{}
Anaesthesia Guidelines for Hepatobiliary Surgery
Pancreaticoduodenectomy (Whipple)–Anaesthetic & ERAS-based Management
Background
Pancreaticoduodenectomy (PD) is the standard curative procedure for malignant or benign peri-ampullary lesions and selected chronic pancreatitis. Reported mortality in high-volume centres is < 3 %, but morbidity remains ≥ 40 % (delayed gastric emptying, pancreatic fistula, haemorrhage, sepsis).
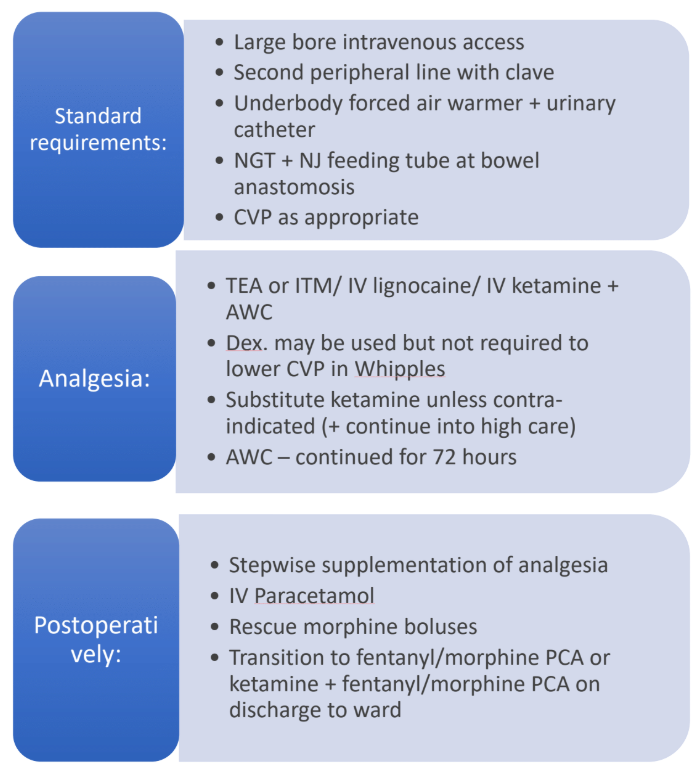
Pre-operative Preparation
| Element | Current best practice |
|---|---|
| Access | Two large-bore (14-16 G) peripheral IVs; rapid-infuser line. Central venous catheter not routine–insert only if major haemorrhage, septic shock or high-dose vasoactive support anticipated. |
| Monitoring | Standard ASA + arterial line before incision; cardiac output monitoring or oesophageal Doppler for goal-directed fluid therapy (GDFT) if ASA ≥ III. |
| Temperature | Under-body forced-air + fluid warmer from induction. |
| Prophylaxis | • Antibiotics: Piperacillin-tazobactam 4.5 g IV ≤ 60 min pre-incision, repeat q4 h; tailor to bile cultures if previous stenting. • LMWH 40 mg SC 2–12 h pre-op; continue for 28 days in malignancy. |
| Tubes & lines | Urinary catheter; nasogastric tube (remove at extubation); surgeon to place naso-jejunal feeding catheter across anastomosis. |
| Optimisation | Implement ERAS interventions: prehabilitation ≥ 3 weeks, smoking/alcohol cessation (≥ 4 weeks), carbohydrate drink 2 h pre-op. Avoid routine pre-op biliary drainage unless bilirubin > 250 µmol L⁻¹ or cholangitis. |
Anaesthetic Technique & Analgesia
Primary Options
| Technique | Evidence summary (2019-2025) | Practical notes |
|---|---|---|
| Thoracic epidural analgesia (TEA) | Improves pain control, preserves pulmonary function, reduces DGE and LOS when functioning ≥ 72 h | Insert T7–T9; 0.1 % bupivacaine + 2 µg mL⁻¹ fentanyl 6–8 mL h⁻¹. Remove once INR < 1.5 & platelet > 80 × 10⁹ L⁻¹. |
| Epidural alternatives | a. Intrathecal morphine (ITM 300 µg)–non-inferior first-24 h analgesia; pruritus common. b. Continuous IV lidocaine (loading 1.5 mg kg⁻¹, then 1–1.5 mg kg⁻¹ h⁻¹ for ≤ 24 h) shortens ileus; higher rates risk > 5 µg mL⁻¹ toxicity ﹙non-linear clearance after 12 h﹚ c. Low-dose ketamine (0.2 mg kg⁻¹ bolus → 0.1–0.2 mg kg⁻¹ h⁻¹ up to 24 h) reduces opioid requirement and postoperative depressive symptoms. |
Choose when TEA contraindicated or centre lacks epidural expertise. |
| Fascial-plane blocks | Bilateral ultrasound-guided erector-spinae plane (ESP) or TAP with catheter provide non-inferior recovery scores vs TEA in recent RCTs; lower hypotension, easier mobilisation. | Bolus 30 mL 0.25 % bupivacaine per side → PCEA 6 mL h⁻¹. |
| Abdominal wound catheter (AWC) | Continuous 0.2 % ropivacaine 5 mL h⁻¹ started in theatre; useful if TEA fails. |
Supplemental Analgesia
- Paracetamol 1 g 6-hourly.
- NSAID/COX-2 inhibitor if creatinine clearance > 60 mL min⁻¹ and anastomosis secure.
- PCA morphine or fentanyl ± ketamine once ITM or TEA weaned.
Intra-operative Management
| Goal | Key actions |
|---|---|
| Haemodynamics & GDFT | Maintain MAP ≥ 65 mmHg with norepinephrine 0.02–0.06 µg kg⁻¹ min⁻¹. Optimise stroke volume using 250 mL balanced crystalloid boluses guided by SVV/PPV (target < 13 %). Avoid total positive balance > 2 L. |
| Fluid composition | Use acetate-buffered crystalloid; add 5 % albumin if > 2 L third-space loss. |
| Blood loss | Cross-match 2–4 units; cell-salvage acceptable unless malignancy breached. TXA 1 g at induction if anticipated EBL > 500 mL. |
| Glycaemic control | Maintain 4–10 mmol L⁻¹; insulin infusion if > 10. |
| PONV prophylaxis | Dexamethasone 8 mg + ondansetron 4 mg; add NK-1 antagonist if ≥ 3 risk factors. |
| Temperature | Active warming to keep core > 36 °C throughout (re-warm before PACU discharge). |
Post-operative Care & ERAS Milestones
| POD | Target | Key points |
|---|---|---|
| 0 | Extubate in theatre / PACU; encourage sitting out of bed; clear fluids as tolerated. | Remove NGT if gastric aspirate < 300 mL 6 h post-op. |
| 1 | Oral liquid diet + chewing gum; urinary catheter out once mobilising. | Early drain amylase. |
| 3 | Evaluate drain fluid; remove drains if amylase < 5000 U L⁻¹ and no leak. | Transition from TEA to oral multimodal; start LMWH prophylaxis if epidural removed. |
| ≤ 5 | Discontinue IV fluids when enteral intake > 60 %. | Aim 750-1000 m ambulation; discharge when pain, nutrition and mobility criteria met (median LOS 7–9 d in high-compliance ERAS pathways). |
Drug-specific Toxicity Limits
| Agent | Safe upper limit | Monitoring |
|---|---|---|
| Bupivacaine | 2 mg kg⁻¹ per 4 h (consider cumulative from TEA + AWC + infiltrations). | Look for perioral numbness, tinnitus, seizure. |
| Lidocaine IV | 1.5 mg kg⁻¹ h⁻¹ (≤ 24 h). Stop if dizziness, metallic taste or serum level > 5 µg mL⁻¹. | 6-hly neuro checks; serum level if infusion > 12 h. |
Updated ERAS® Pancreatic Surgery Key Recommendations (2019 → 2024 evidence)
| Domain | 2024 evidence change |
|---|---|
| Prehabilitation | ≥ 4-week supervised programme decreases serious complications (Clavien ≥ III) by 8 %. |
| Immunonutrition | Remains not recommended–network meta-analysis 2023 showed no reduction in infectious complications. |
| Minimally invasive PD | Robotic PD acceptable in centres performing ≥ 20 cases yr⁻¹ with < 5 % 90-day mortality; still awaiting RCTs. |
| Analgesia | Continuous ESP or TAP catheters are acceptable alternatives to TEA within multimodal, opioid-sparing protocols. |
| Fluid therapy | GDFT with dynamic indices superior to liberal or fixed restrictive regimens. |
| Somatostatin analogues | Updated meta-analysis 2023: reduce clinically relevant POPF only in high-risk (soft pancreas, small duct)–selective use recommended. |
Links
Liver physiology and pathology
Past Exam Questions
Perioperative Nutrition for a Patient Undergoing a Pancreaticoduodenectomy
A 68-year-old female presents for a pancreaticoduodenectomy (Whipple’s procedure) for cancer of the head of the pancreas. She has no significant comorbidities. The procedure is booked for the following week (8 days after the consultation). Describe your approach to her perioperative nutrition.
a) Pre-operatively. (4)
b) Intra-operatively. (2)
c) Post-operatively. (4)
References
- Melloul, E., Lassen, K., Roulin, D., Grass, F., Périnel, J., Adham, M., … & Demartines, N. (2020). Guidelines for perioperative care for pancreatoduodenectomy: enhanced recovery after surgery (eras) recommendations 2019. World Journal of Surgery, 44(7), 2056-2084. https://doi.org/10.1007/s00268-020-05462-w
- Batty, D. (2021). Guidelines for Anaesthesia for Hepatobiliary Surgery: GSH and UCTPAH. Unpublished institutional guidelines, Groote Schuur Hospital and University of Cape Town Private Academic Hospital.
- Melloul E, et al. ERAS Society guidelines for pancreatoduodenectomy–2019. World J Surg. 2021;45:1341-62. ahpba.org
- Khan S, et al. Comparative efficacy of peri-operative lidocaine infusion versus thoracic epidural for major abdominal surgery. Pain Pract. 2025;25:112-22. pmc.ncbi.nlm.nih.gov
- Liu C, et al. Systematic review of IV vs intraperitoneal lidocaine for abdominal surgery. Reg Anesth Pain Med. 2024;49:88-99. pubmed.ncbi.nlm.nih.gov
- Zhang H, et al. Intrathecal morphine vs epidural for open pancreatic surgery: meta-analysis. Anaesthesia. 2024;79:55-66. pmc.ncbi.nlm.nih.gov
- Yu L, et al. Continuous erector-spinae plane block non-inferior to TEA for recovery after thoracoscopic surgery. J Clin Anesth. 2025;85:111019. pubmed.ncbi.nlm.nih.gov
- Chen Y, et al. Esketamine improves mood and analgesia after pancreatoduodenectomy: RCT. BMC Anesthesiol. 2023;23:331. pmc.ncbi.nlm.nih.gov
- French ERAS-PD Collaborative. Goal-directed fluid therapy reduces major morbidity after Whipple. Ann Surg. 2024;280:e164-e172. pmc.ncbi.nlm.nih.gov
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “a4eebee6-1c07-41ce-a10d-654fb255154c”



