- Introduction
- Dialysis Prescription in AKI
- Anaesthesia and Surgical Techniques for PD Catheter Insertion
Introduction
Comparison of Peritoneal Dialysis and Haemofiltration
Peritoneal Dialysis:
- Cheaper.
- Utilizes a biocompatible membrane.
- Provides cardiovascular stability.
- Does not require vascular access.
- Avoids the need for anticoagulation.
- No specialized equipment or nursing required.
- Facilitates more rapid recovery of renal function compared to haemodialysis.
- Allows easy transition to long-term PD.
Haemofiltration:
- Greater control over ultrafiltration rate.
- Suitable for patients who have undergone laparotomy.
Solute Removal and Fluid Removal in Peritoneal Dialysis
Solute Removal Mechanisms:
- Diffusion: Selective movement of small solutes (e.g., potassium, urea, creatinine) down a concentration gradient across a semipermeable membrane.
- Example: Like tea diffusing from a tea bag into water.
- Convection: Non-selective movement of larger molecules (e.g., proteins) with water, also known as solute drag.
- Example: Squeezing a tea bag forces tea out with water.
Process:
- Dialysis fluid is instilled into the abdominal cavity, where small solutes move from serum into the peritoneal fluid due to concentration gradients.
- Water moves into the peritoneal cavity by osmosis, dragging larger molecules with it.
- Replacing equilibrated dialysis fluid with fresh solution is necessary to maintain the concentration gradient.
Fluid Removal Mechanism:
- Achieved by osmosis, driven by glucose in the PD fluid.
- Higher glucose concentration increases fluid removal.
- Prolonged dwell times result in glucose diffusion back into the patient, reducing osmotic gradient and causing fluid reabsorption.
- Increased fluid removal requires higher glucose concentrations or shorter cycle times (minimum one hour).
- Solutes such as urea, creatinine, phosphate, and glucose reach equilibrium at different rates during PD.
- Urea equilibrates most rapidly, followed by creatinine, phosphate, and β₂-microglobulin. Glucose concentration in dialysate decreases over time due to diffusion into the serum.
Peritoneal Membrane Structure
- Mesothelium:
- Protective barrier with villous projections, increasing the membrane surface area (~20m²).
- No role in solute flow regulation.
- Interstitium: Connective matrix maintaining membrane integrity.
- Capillaries: Serve as the semipermeable membrane for solute and water exchange.
Devices for Peritoneal Dialysis
Rigid Catheter:
- Plastic catheter inserted subumbilically.
- Advantages:
- Easy insertion.
- Requires minimal training.
- Cost-effective.
- Disadvantages:
- Narrow lumen leading to slow dialysate flow and frequent blockages.
- Higher risk of leakage, hemorrhage, and peritonitis.
- Should only be used if flexible catheters are unavailable.
Flexible Tenckhoff Catheter:
- Made of silastic with Dacron cuffs, available in straight or coiled designs.
- Inserted via a percutaneous or surgical approach.
- Advantages:
- Higher efficiency and fewer complications compared to rigid catheters.
- Tunneling under the skin reduces leakage and infection risks.
- Contraindications for percutaneous insertion include:
- Midline surgical scars.
- Previous abdominal tuberculosis.
- Complex appendectomy or cholecystectomy.
Dialysis Prescription in AKI
Key Considerations:
- Fluid Overload:
- Use 3.86% glucose solution for pulmonary edema or severe fluid overload.
- Use more frequent fluid exchanges for higher fluid removal.
- Hyperkalaemia/Acidosis:
- Corrected by increasing the frequency of fluid exchanges.
- Dehydration:
- Use 1.36% glucose solution.
Cycle Time:
- Minimum one hour to maximize effective dialysis time.
Evidence For Peritoneal Dialysis in AKI
- Studies from Brazil and India indicate comparable urea clearances to intermittent haemodialysis, with earlier recovery of renal function in PD patients (e.g., by 3 days).
- Cytokine clearance may be greater with PD, though evidence is limited.
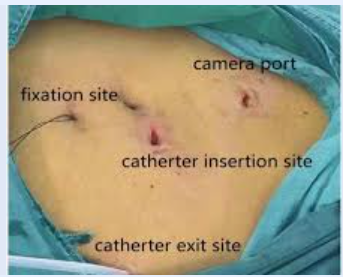
Anaesthesia and Surgical Techniques for PD Catheter Insertion
General Anesthesia (GA):
- Induction:
- Propofol: 1–2 mg/kg intravenously.
- Fentanyl: 1–2 µg/kg intravenously.
- Cisatracurium: 0.15 mg/kg intravenously for tracheal intubation.
- Maintenance:
- Oxygen and air with either:
- Sevoflurane or Desflurane: Titrated to an age-adjusted Minimum Alveolar Concentration (MAC) of 0.8–1.1.
- Oxygen and air with either:
Local Anesthesia (LA):
- Sedation:
- Initiated after applying monitors.
- Midazolam: 0.015 mg/kg intravenously.
- Fentanyl: 1–2 µg/kg intravenously.
- Remifentanil (optional): 0.01–0.1 µg/kg/min infusion.
- Propofol: Administered as:
- Intermittent boluses of 10–20 mg.
- Continuous infusion at 25–150 µg/kg/min.
- Titrated to maintain a sedation level of 3–4 on the Observer’s Assessment of Alertness/Sedation (OAA/S) scale.
- Supplemental Oxygen: Delivered via facemask to all patients.
- Hemodynamic Fluctuations: Managed with small doses of vasopressors or vasodilators.
Surgical Technique:
- Local Anesthesia: Achieved using 1% lidocaine to infiltrate the soft tissue and peritoneum.
- Pneumoperitoneum:
- Nitrous Oxide (N₂O): Used for LA group.
- Carbon Dioxide (CO₂): Used for GA group.
- Insufflation pressure: Maximum of 12 mmHg.
Postoperative Care:
- All patients were transported to the Post-Anesthesia Care Unit (PACU) for monitoring following surgery.
Key Points from Comparative Studies of LA vs. GA
- LA Group:
- Shorter procedure and recovery times.
- Lower perioperative risk for high-risk patients.
- Equivalent PACU scores on discharge compared to GA.
- GA Group:
- Necessary for more complex cases with prior abdominal surgery or need for extensive adhesiolysis.
Both techniques are safe and effective, with nearly 45% of patients being suitable for LA.
Links
References:
- Liu X, Zuo X, Heng X, Abreu Z, Penner T, et al. (2017) Anesthesia Considerations for Insertion of the Peritoneal Dialysis Catheter. J Clin Nephrol Ren Care 3:028. doi.org/10.23937/2572-3286.1510028
- Maio R, Figueiredo N, Costa P (2008) Laparoscopic placement of Tenckhoff catheters for peritoneal dialysis: a safe, effective, and reproducible procedure. Perit Dial Int 28: 170-173.](https://www.ncbi.nlm.nih.gov/pubmed/18332453)
- Manouras AJ, Kekis PB, Stamou KM, Konstadoulakis MM, Apostolidis NS (2004) Laparoscopic placement of Oreopoulos-Zellerman catheters in CAPD patients. Perit Dial Int 24: 252-255.](https://www.ncbi.nlm.nih.gov/pubmed/15185773)
id: “6d63134c-6021-48dc-b48b-24c5c99a7683”



