- Mediastinal Anatomy & Pathology
- Anaesthesia for Patients with Mediastinal Masses
- Crisis Management Algorithms
- Special Tumour-Specific Issues
{}
Mediastinal Anatomy & Pathology
Contemporary Compartment Model
The International Thymic Malignancy Interest Group (ITMIG) describes three radiological compartments on cross-sectional imaging:
| Compartment | Boundaries | Typical Lesions |
|---|---|---|
| Pre-vascular (Anterior) | Sternum → anterior pericardium | Thymoma, thymic carcinoma, germ-cell tumours, lymphoma, ectopic thyroid or parathyroid, teratoma |
| Visceral (Middle) | Anterior → posterior pericardium | Bronchogenic or pericardial cysts, lymphadenopathy, tracheal and oesophageal tumours, vascular anomalies |
| Paravertebral (Posterior) | Posterior pericardium → vertebral bodies & thoracic outlet | Neurogenic tumours (schwannoma, neuroblastoma), meningocoeles, paravertebral abscess |
The traditional “anterior–middle–posterior” surgical division at the T4/5 angle of Louis remains useful for historical literature but should be cross-referenced with ITMIG terminology for modern imaging reports.
Epidemiology & Tumour Biology
- Adults: Lymphoma and thymic epithelial tumours predominate.
- Children: Neurogenic tumours and lymphomas are common.
- Rapidly proliferating lymphomas may respond dramatically to steroids or chemotherapy; biopsy before treatment is therefore essential unless airway compromise necessitates urgent debulking or stenting.
Anaesthesia for Patients with Mediastinal Masses
Key Principles
- Spontaneous breathing preserves airway patency and venous return.
- Muscle relaxants: Use only when airway beyond lesion is secured and haemodynamics stable. Sugammadex allows rapid reversal if collapse occurs.
- Positioning: Semi-Fowler’s or lateral decubitus may be life-saving; rehearse roll-over drills with team.
- Intra-operative monitoring:
- Two pulse oximeters (right hand, lower limb) to detect great-vessel compression.
- Arterial line (preferably right radial) before induction in intermediate/high-risk cases.
- TOE or transthoracic echo for dynamic PA/cardiac compression during resection.
- Vascular access: At least one large-bore line below the diaphragm in any patient with suspected SVC obstruction.
- Cardiopulmonary bypass / ECMO: 2024 EACTS–EACTAIC guidelines support pre-emptive femoral cannulation in selected high-risk adults and all high-risk children.
Structured Pre-operative Assessment
| Domain | Red-flag findings |
|---|---|
| Symptoms | Orthopnoea, stridor, syncope, exertional or positional dyspnoea/cough, head/neck plethora (SVC syndrome), constitutional B-symptoms in lymphoma |
| Imaging | >50 % tracheal lumen reduction, mainstem or carinal compression, PA or cardiac chamber impingement, pericardial effusion, compression of SVC/innominate/IVC |
| Functional tests | Supine fall in peak expiratory flow >40 % or inability to tolerate supine CT; flow-volume loops poorly predict risk and are no longer recommended as sole screen |
| Cardiorespiratory reserve | Echo for PA pressures & ventricular compression; cardiopulmonary exercise testing if time permits |
| Paraneoplastic Syndromes | Myasthenia Gravis (MG) with thymic mass. |
- High-risk phenotype: any airway/cardiovascular red flag plus symptomatic orthopnoea or syncope.
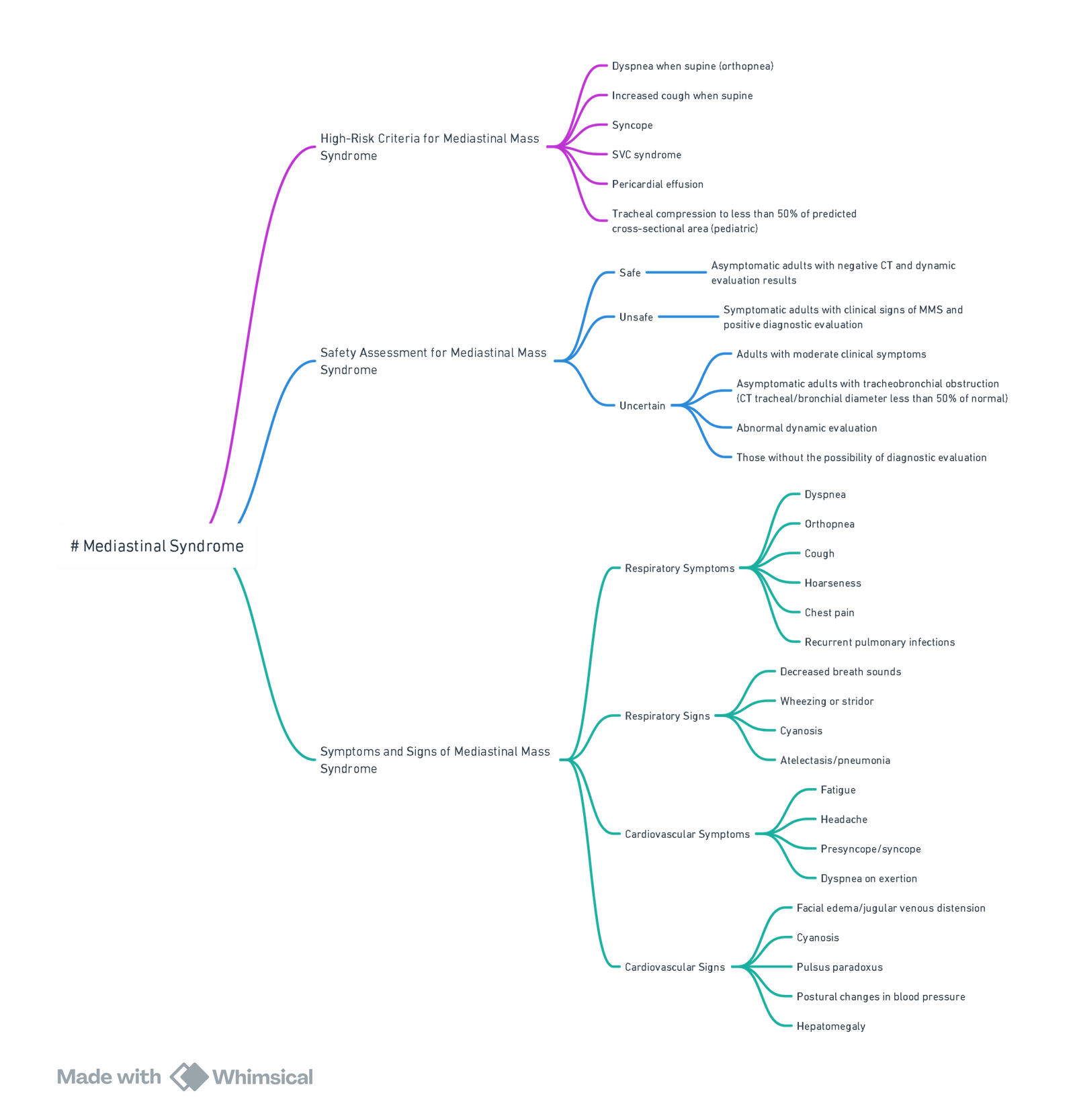
Mediastinal Syndrome
View or edit this diagram in Whimsical.
Investigations
- Chest X-ray (CXR).
- CT Scan: Evaluate the size, site, severity, and degree of compression of vital structures.
- Lung Function Tests: Generally not useful.
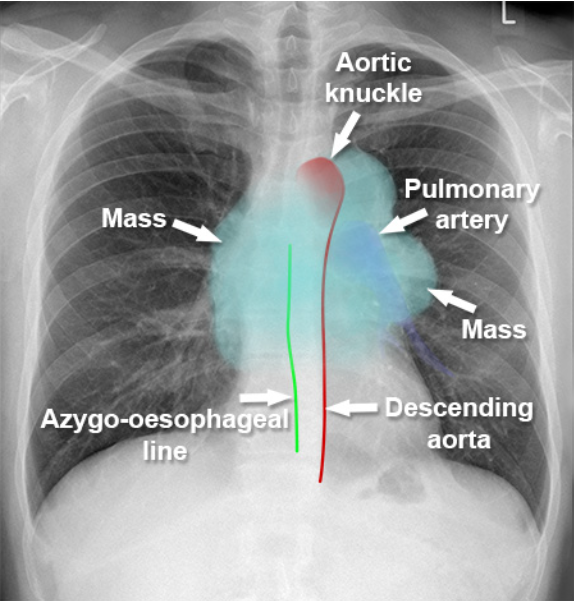
Localization of Mediastinal Mass
- General CXR signs
- Unlike lung lesions, a mediastinal mass will not contain air bronchograms.
- The margins with the lung will be obtuse.
- Mediastinal lines (azygoesophageal recess, anterior and posterior junction lines) will be disrupted.
- There can be associated spinal, costal or sternal abnormalities.
- Anterior: Hilum overlay sign (mostly), silhouette sign (heart border obliterated)
- Middle: Displaced azygoesophageal recess on the right; pseudoparavertebral line on the left
- Posterior: Cervicothoracic Sign
Pathophysiological Changes During GA
| Mechanism | Effect | Mitigation |
|---|---|---|
| Loss of gravity assistance in supine | Tumour falls posteriorly → airway/SVC compression | Keep head-up; delay supine until airway secured |
| Positive-pressure ventilation | Abolishes negative pleural pressure → dynamic airway collapse; reduces venous return | Use low driving pressures, permissive hypercapnia, or pressure-support with spontaneous effort |
| Anaesthetic muscle relaxation | Reduces thoracic muscle tone & tracheal cartilaginous support | Defer relaxant; consider sugammadex-enabled “test” dose |
| Enlarged central blood volume supine | Tumour engorgement ↑ | Position optimisation; minimal fluids until haemodynamics assessed |
| Surgical manipulation | Oedema, bleeding within mass | Communicate; pause ventilation & adjust FiO₂ before specimen extraction |
Decision Making
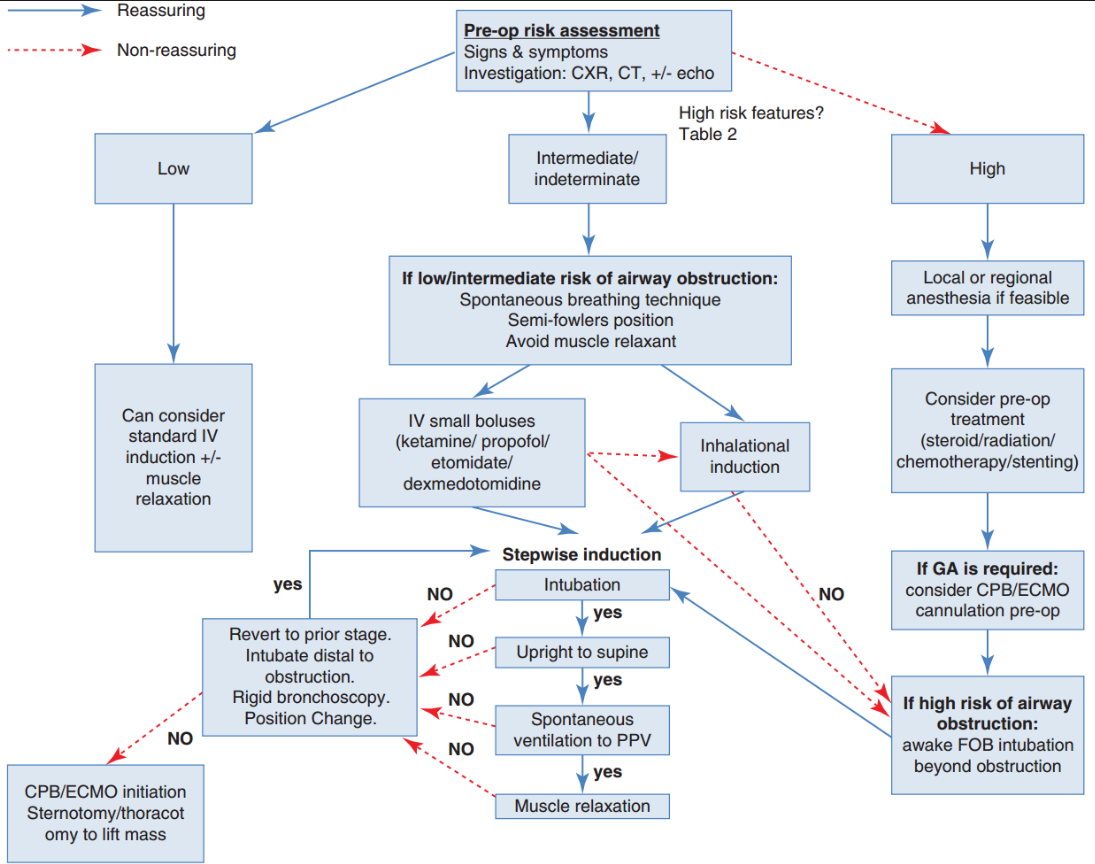
- Stepwise Induction:
- Induction and intubation should follow a stepwise approach, ensuring verification of adequate ventilation and circulation at each stage before advancing.
- The use of short-acting medications is recommended to enable a return to the previously tolerated state, such as spontaneous ventilation.
Options for Induction
- No induction:
- Local or regional anesthesia with or without sedation.
- Awake fiber-optic intubation:
- Placement of the endotracheal tube (ETT) beyond the area of compression or stenosis.
- Induction follows after securing the airway.
- Maintenance of spontaneous ventilation:
- Utilize small boluses of intravenous anesthetics such as:
- Ketamine,
- Propofol, or
- Etomidate.
- Alternatively, perform inhalational induction.
- Utilize small boluses of intravenous anesthetics such as:
- Standard intravenous induction:
- Can be performed with or without the use of muscle relaxation.
Preoperative Risk Assessment
- Evaluate signs and symptoms.
- Conduct investigations:
- Chest X-ray (CXR)
- Computed tomography (CT)
- ± Echocardiography (Echo)
- Assess for high-risk features (see above):
- Non-reassuring findings → High-risk pathway.
- Reassuring findings → Low-risk pathway.
High-Risk Criteria
- Dyspnea when supine (orthopnea)
- Increased cough when supine
- Syncope
- Superior vena cava (SVC) syndrome
- Pericardial effusion
- Tracheal compression to <50% of predicted cross-sectional area (pediatric)
Peri-operative Strategy Matrix
| Risk tier | Airway & ventilation | Induction plan | Rescue readiness |
|---|---|---|---|
| Low (asymptomatic, <50 % compression) | Standard IV induction (propofol + short-acting opioid) ± NMB; volume-controlled ventilation | Supine tolerated | Standard difficult-airway cart |
| Intermediate (positional symptoms or borderline imaging) | Maintain spontaneous ventilation until airway secured beyond lesion (reinforced tube / long SLT) | Titrated IV or inhalational induction without muscle relaxant; patient sat 30 ° | Rigid bronchoscope & jet ventilation immediately available |
| High (orthopnoea, >50 % airway/PA/SVC compression, pericardial effusion) | Avoid GA if possible: local/neuraxial, MAC or EBUS under sedation | If GA essential → awake fibre-optic intubation distal to mass, femoro-femoral ECMO/CPB cannulation under LA before induction | Primed ECMO circuit, sternotomy team scrubbed |
Crisis Management Algorithms
Airway Obstruction
- Call “mass collapse” code.
- Re-establish spontaneous breathing (cease relaxant, give sugammadex if rocuronium).
- Rigid bronchoscopy & jet ventilation.
- Re-position (lateral/ prone) or reopen sternum to elevate mass.
- If ventilation impossible → initiate pre-cannulated ECMO.
Cardiovascular Collapse
- Elevate legs, fluid bolus, phenylephrine/epinephrine.
- Turn patient lateral or prone.
- If external compression persists → sternotomy or ECMO
SVC Syndrome Intra-operatively
- Maintain head-up.
- Administer diuretics ± steroids.
- Avoid Trendelenburg and excessive IV fluids via upper-body lines.
Post-procedure chest radiograph and ultrasound screen for pneumothorax, hemothorax and pericardial effusion remain standard.
Special Tumour-Specific Issues
| Tumour | Anaesthetic implications |
|---|---|
| Thymoma | 30 % coexistence with myasthenia gravis → unpredictable response to NMB; avoid long-acting relaxants; consider low-dose rocuronium with neuromuscular monitoring; anticipate post-op ventilatory support |
| Germ-cell tumours | Bleomycin exposure demands FiO₂ <0.30 where feasible and judicious fluids to minimise pulmonary toxicity |
| Non-small-cell lung cancer | Eaton–Lambert myasthenic syndrome causes ↑ sensitivity to both depolarising & non-depolarising blockers; sugammadex reversal still effective but requires quantitative monitoring |
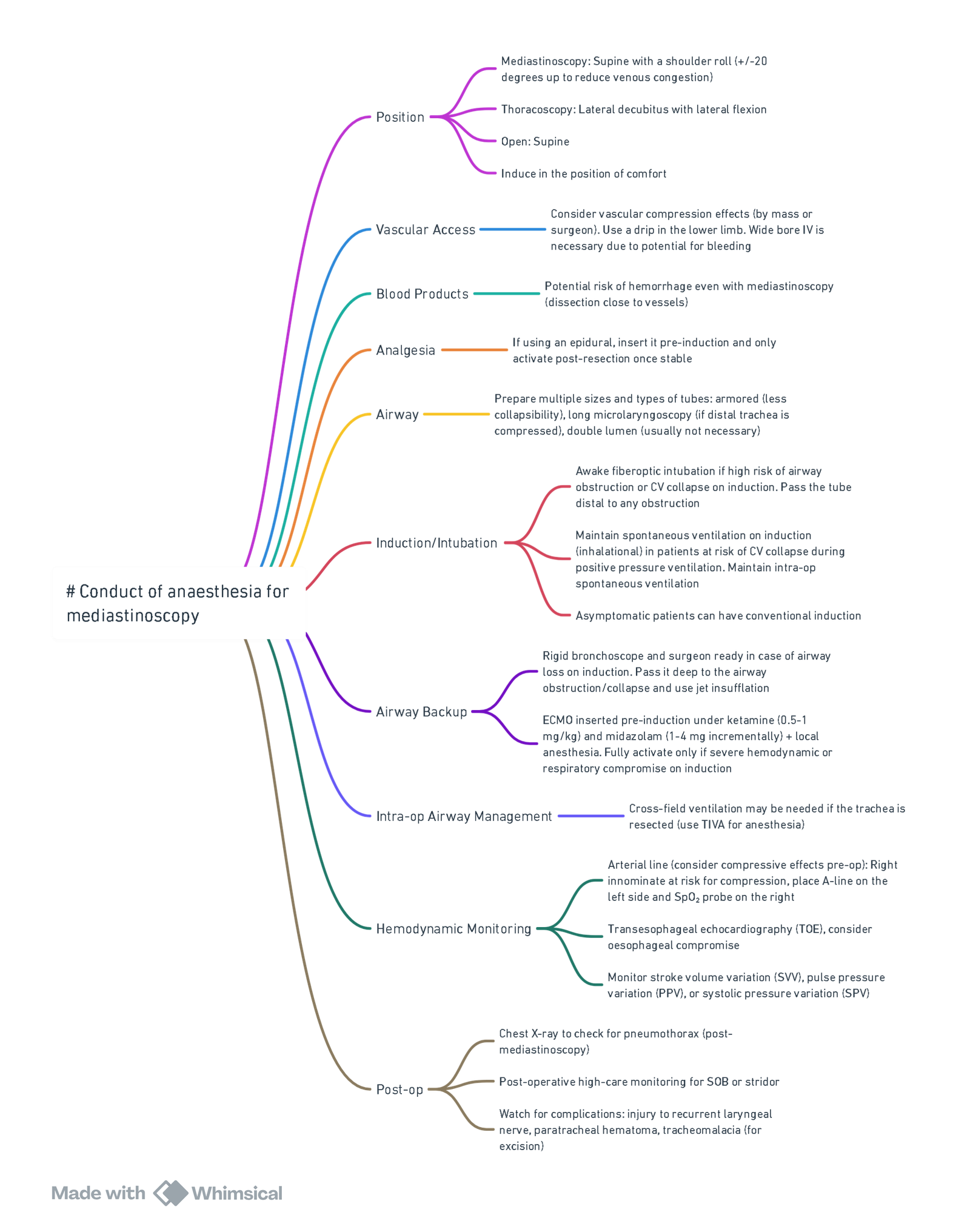
Mediastinoscopy & Tissue Acquisition
| Approach | Target nodal stations | Key anaesthetic considerations |
|---|---|---|
| Cervical (video-assisted remains gold standard) | 2R/L, 4R/L, ±7 | Table-mounted scope causes cephalad tracheal deviation; brief pause ventilation when scope traverses carina; watch right-hand SpO₂ for brachiocephalic compression. |
| Left anterior (Chamberlain) | 5, 6 | Requires left second intercostal incision; lung isolation not mandatory. |
| Endobronchial ultrasound-guided TBNA (EBUS-TBNA) | 2, 4, 7, 10, 11 | Frequently supplants surgical mediastinoscopy; can be performed awake, under deep sedation, or GA with LMA. |
- Complication rate remains <3 %; major bleeding is rare but catastrophic—large-bore arterial line and two large-bore IVs sited below the diaphragm are advised.
- Relative contra-indications mirror those in current thoracic society guidance: prior mediastinoscopy, SVC syndrome, large aortic aneurysm, severe tracheal deviation or calcified nodes abutting major vessels.
View or edit this diagram in Whimsical.
Anterior Mediastinal Masses in Children
- Large anterior mediastinal masses (AMMs) in children can produce critical airway, cardiovascular and venous outflow obstruction that may be unmasked—or aggravated—by general anaesthesia (GA). A structured, team-based pathway lowers peri-operative morbidity and mortality.
Multidisciplinary Planning
| Core team | Early actions |
|---|---|
| Paediatric oncologist | Decide if tissue diagnosis can be delayed while tumour burden is reduced with steroids, chemotherapy or radiotherapy. |
| Paediatric surgeon / interventional radiologist | Select least invasive, safest biopsy route (e.g. ultrasound-guided supraclavicular node, CT-guided posterior core, endobronchial ultrasound). |
| Consultant paediatric anaesthetist | Lead risk assessment; formulate airway, vascular access, rescue and extracorporeal support plans. |
| Paediatric intensivist & ECMO team | Prepare cannulation strategy (usually femoro-femoral veno-venous) for high-risk cases. |
| Family | Discuss risks, possible awake/sedation techniques, potential need for postoperative ventilation or ECMO. |
Pre-operative Assessment
History & Examination
- Positional respiratory symptoms: cough, orthopnoea, stridor, wheeze—especially when supine.
- Cardiovascular signs: syncope, facial/upper-body oedema, distended neck veins (suggesting superior vena cava obstruction).
- Tolerance of lying flat during CT or echocardiography is a pragmatic bedside test.
Imaging & Functional Tests
| Modality | Utility | High-risk thresholds* |
|---|---|---|
| Chest radiograph | Screening; mediastinal widening; tracheal deviation | — |
| Contrast CT chest (supine &—if possible—prone or lateral) | Defines airway and vessel calibre; quantifies cross-sectional area (CSA) | – Tracheal CSA <50 % (any age) – Tracheal CSA <70 % and bronchial/great-vessel compression |
| Echocardiography (transthoracic) | Detects pericardial effusion, cardiac chamber/PA compression, ventricular function | Effusion >10 mm or tamponade physiology; PA or ventricular compression |
| MRI (sedation-free if child tolerant) | Superior for vascular invasion and dynamic airway collapse; avoids ionising radiation | Same thresholds as CT |
| Flow-volume loops | Not recommended (high false-negative rate). | — |
Adapted from contemporary paediatric AMM guidelines (2024–25).
Risk Stratification
| Tier | Clinical & imaging profile | Recommended location for procedure |
|---|---|---|
| Low | Asymptomatic; tracheal CSA ≥70 %; no cardiac or great-vessel compromise | Day-case theatre with consultant anaesthetist |
| Intermediate | Positional symptoms or borderline imaging criteria | Paediatric tertiary centre with immediate rigid bronchoscopy & ECMO access |
| High | Orthopnoea, stridor, syncope, SVC syndrome or any high-risk imaging feature | Hybrid theatre/ICU with ECMO team scrubbed and femoral vessels pre-wired under local anaesthesia (LA) |
Anaesthetic Techniques
Local Anaesthesia (± Sedation)
- Preferred for superficial lymph-node biopsies.
- Sedation strategy: dexmedetomidine 1 µg kg⁻¹ over 10 min then 0.2–1 µg kg⁻¹ h⁻¹; titrated ketamine 0.5–1 mg kg⁻¹ IV boluses maintain airway tone.
- Back-up plan: transition to GA without neuromuscular blockade (NMB) if distress or movement compromises accuracy
General Anaesthesia
- Shared principles for all GA in AMM
- Secure lower-body intravenous access before induction (SVC may obstruct upper-body lines).
- Maintain spontaneous ventilation (SV) until airway distal to lesion is secured.
- Avoid NMB until safe. If required, use low-dose rocuronium (0.3–0.4 mg kg⁻¹) with quantitative monitoring; be prepared to reverse with sugammadex.
- Induction options
- Inhalational sevoflurane (child upright or semi-Fowler).
- Titrated IV propofol 1–2 mg kg⁻¹ in older children who tolerate cannulation awake.
- Airway equipment immediately available: reinforced single-lumen tubes of several sizes, long (microlaryngeal) tubes, rigid bronchoscope with jet ventilation, paediatric fibre-optic bronchoscope.
- Positioning drills rehearsed (lateral, prone, sitting) to relieve compression.
Difficult-airway Algorithm (high-risk children)
- Cannot ventilate ± cannot oxygenate despite SV
- –> Return to previously tolerated position → Rigid bronchoscope → Advance beyond obstruction → Jet ventilation → If still impossible → Activate pre-cannulated veno-venous ECMO.
Intra-operative Monitoring & Rescue
- Continuous capnography & flow–volume trend detect rising resistance/trapping.
- Pulse oximeters: one upper limb, one lower limb to show SVC compression.
- Arterial line (right radial) for all intermediate/high-risk cases; femoral preferred if right upper-body access compromised.
- Transoesophageal echo if significant cardiac compression suspected or during mass resection.
- Rescue manoeuvres summarised below:
| Complication | Immediate actions |
|---|---|
| Airway obstruction | 100 % O₂, CPAP 10–15 cmH₂O, reposition, rigid bronchoscope, intubate distal to lesion, consider one-lung ventilation |
| Cardiovascular collapse | Trendelenburg or lateral decubitus, fluid 10 mL kg⁻¹, vaso-pressors (phenylephrine 1 µg kg⁻¹ IV), lighten anaesthesia, emergent sternotomy or ECMO |
| SVC syndrome (acute) | Head-up 30°, minimise IV fluid via upper lines, continue lower-body infusions, consider mannitol/loop diuretic, keep airway pressure low |
Extracorporeal Support
- Pre-emptive femoro-femoral veno-venous ECMO cannulation under LA is recommended by several tertiary centres for symptomatic high-risk patients (tracheal CSA <50 % or great-vessel compression with syncope).
- Rescue ECMO after complete cardiorespiratory collapse carries <30 % survival; thus emphasis on prophylactic cannulation where feasible.
Post-operative Care
- High-risk or incompletely resected masses: elective ICU admission, maintain head-up, extubate only when fully awake and breathing comfortably upright.
- Monitoring: serial chest radiograph and echocardiography for delayed effusion or pneumothorax; electrolytes & urate for tumour lysis syndrome (TLS).
- Pain management: paracetamol and low-dose opioid; avoid high thoracic epidural unless coagulation normal.
Pathology & Onco-anaesthetic Considerations
| Diagnosis | Frequency | Anaesthetic implications |
|---|---|---|
| Hodgkin lymphoma | Commonest paediatric AMM | Rapid steroid response may shrink mass—balance against loss of diagnostic tissue. |
| Non-Hodgkin lymphoma | Second most common | High TLS risk; hydrate 3 mL kg⁻¹ h⁻¹ and monitor potassium, phosphate, urate. |
| T-cell acute lymphoblastic leukaemia | ~10 % | Profound TLS potential; avoid succinylcholine (hyperkalaemia). |
| Germ-cell tumours | ~5 % | α-fetoprotein / β-hCG support diagnosis; bleomycin exposure → limit FiO₂ to ≤0.30. |
| Neurogenic tumours, cysts, vascular malformations | Uncommon | May encroach posteriorly; consider double-lumen tube or bronchial blocker if thoracotomy. |
Links
References:
1. Sarkiss M, Jimenez CA. The evolution of anesthesia management of patients with anterior mediastinal mass. Mediastinum. 2023 Mar 6;7:16. doi: 10.21037/med-22-37. PMID: 37261097; PMCID: PMC10226893.
2. McLeod, M., & Dobbie, M. (2019). _Anterior mediastinal masses in children_. BJA Education, 19(1), 21–26. https://doi.org/10.1016/j.bjae.2018.10.001
3. Subramanyam R, et al. _Anesthetic risk with large mediastinal masses: a management framework._ Ann Thorac Surg. 2025. PubMed
4. Mohanty CR, et al. _Perioperative management of patients with mediastinal mass syndrome._ Curr Opin Anaesthesiol. 2025. PubMed
5. Tan J, et al. Anaesthesia for children with anterior mediastinal masses. _Pediatr Anaesth_ 2021;31:1192-1205. Renaissance School of Medicine
6. Anterior Mediastinal Mass Guideline, Adelaide Children’s Hospital Paediatric Critical Care Group (ACH PCCG). 2024. ACH PCCG
7. Rutledge A, et al. Implementation of an anterior mediastinal mass pathway to expedite biopsy in children. _Pediatr Qual Saf_ 2024;9:e672. Lippincott Journals
8. Subramanyam R, et al. Anaesthetic risk with large mediastinal masses: a management framework. _Ann Thorac Surg_ 2024;118:556-565. Annals of Thoracic Surgery
9. Sheffield Children’s Hospital. Emergency management of a mediastinal mass guideline (Version 2.0). January 2025. Sheffield Children’s NHS Trust
10. Klijian AS, et al. _Challenges and pitfalls in the perioperative management of mediastinal mass syndrome: an up-to-date review._ Anaesth Intensive Care. 2024. Termedia
11. EACTS/EACTAIC/EBCP. _Guidelines on cardiopulmonary bypass for adult cardiac surgery._ BJA. 2024. bjanaesthesia.org
12. Rutten A, et al. _Use of rocuronium and sugammadex during video-assisted mediastinal procedures: a prospective study._ BJA. 2022. bjanaesthesia.org
13. 1. Mediastinal masses and anaesthesia for mediastinoscopy-Wits refresher 2014. Prof. J Swanevelder
Summaries:
Mediastinal anatomy
Anaesthesia for mediastinal surgery
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “e0c09d55-d6a4-40d1-bcc4-e9218c96cb97”



