- Thyroid
- Parathyroid
- Adrenal Gland
- Carcinoid Syndrome
- Multiple Endocrine Neoplasia (MEN)
- Acromegaly
- Anorexia Nervosa
- Diabetes Insipidus (DI)
- SIADH
- Panhypopituitarism
- Porphyria
- Links
- Past Exam Questions
- Anaesthesia for Acute Porphyria
{}
Thyroid
Key Points
- Thyroid disease is common and has major peri-operative implications for cardiovascular, respiratory, metabolic and neuromuscular function.
- Aim to render all elective surgical patients clinically and biochemically euthyroid.
- Large goitres rarely cause impossible intubation, but distorted anatomy and tracheomalacia increase the risk of peri-extubation airway failure and neck haematoma.
- High-flow nasal oxygen (HFNO/THRIVE) improves apnoeic oxygenation during induction and extubation but does not replace a secured airway.
- Intra-operative nerve monitoring (IONM) should be considered for redo surgery, malignancy, bilateral dissection or pre-existing vocal-cord palsy. Continuous IONM requires avoidance of neuromuscular blockade after initial mapping.
Thyroid Physiology
Synthesis and Regulation
| Step | Process | Key enzyme/transport | Notes |
|---|---|---|---|
| 1 | Iodide trapping | Na⁺/I⁻ symporter | Blocked by perchlorate/thiocyanate |
| 2 | Oxidation & organification | Thyroid peroxidase (TPO) | Forms monoiodotyrosine (MIT) & di-iodotyrosine (DIT) |
| 3 | Coupling | TPO | DIT + DIT → T4; DIT + MIT → T3 |
| 4 | Storage | Thyroglobulin in colloid | Months of reserve |
| 5 | Release | Endocytosis & lysosomal cleavage | TSH-dependent |
| 6 | Peripheral conversion | 5ʹ-deiodinase types 1 & 2 | Converts ~80 % of circulating T3 |
- Feedback axis: Hypothalamic TRH → pituitary TSH → thyroid; negative feedback by free T4/T3. Iodine deficiency, illness, drugs (amiodarone, steroids) and pregnancy alter this axis.
T3 versus T4
| Hormone | Fraction of glandular output | Degree of protein binding | Relative potency | Plasma half-life |
|---|---|---|---|---|
| T3 | ≈ 10 % | Lower than T4 (binds mainly to albumin & transthyretin) | 3–5 × T4 | ≈ 24 h |
| T4 | ≈ 90 % | ~75 % bound to thyroxine-binding globulin | Pro-hormone (precursor to T3) | ≈ 7 d |
Physiological Effects of T3
- Increased_=: β-adrenergic receptor density, myocardial α-myosin heavy chain, basal metabolic rate, thermogenesis, ventilation, bone turnover, neuromuscular excitability.
- Critical for: fetal brain myelination, linear growth, reproductive function.
Interpretation of Thyroid Function Tests
- Euthyroid:
- TSH: 0.4–4.5 mU l⁻¹; Free T4: 9–25 pmol l⁻¹; Free T3: 3.5–7.8 pmol l⁻¹
| Pattern | Likely diagnosis |
|---|---|
| ↑TSH, normal FT4 | Subclinical hypothyroidism |
| ↓TSH, normal FT4/FT3 | Subclinical hyperthyroidism, exogenous T4 |
| ↑TSH, ↓FT4/FT3 | Primary hypothyroidism (autoimmune, post-radio-iodine, post-surgery) |
| ↓/normal TSH, ↓FT4/FT3 | Secondary (pituitary) hypothyroidism, severe illness (“euthyroid sick”) |
| Normal/↑TSH, ↑FT4/FT3 | TSH-secreting adenoma, thyroid hormone resistance, amiodarone effect |
| ↓TSH, ↑FT4/FT3 | Primary hyperthyroidism (Graves’, toxic MNG, adenoma, thyroiditis) |
Anaesthesia for Thyroidectomy
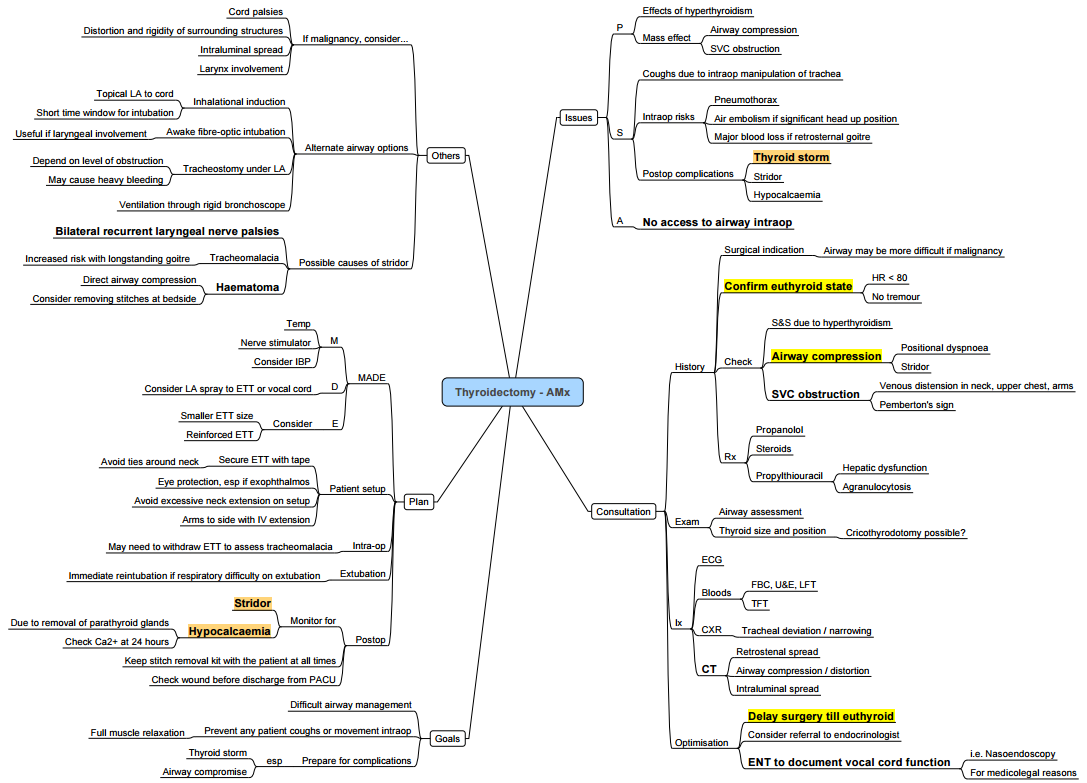
Pre-operative Assessment
- Thyroid status–ensure euthyroid; if hyperthyroid postpone 6–8 weeks or optimise with β-blocker ± thionamide ± iodine ± steroid.
- Goitre assessment
- Duration > 5 y or tracheal diameter < 8 mm → suspect tracheomalacia.
- Positional dyspnoea, orthopnoea, dysphagia or voice change mandate CT neck/chest and nasendoscopy.
- Pemberton’s sign or venous congestion → consider SVC obstruction; site IV access below diaphragm.
- Airway imaging–CT provides accurate tracheal calibre and tube size prediction; CXR overestimates diameter.
- Cardiorespiratory–ECG, echocardiography if AF, heart failure or pulmonary hypertension.
- Laboratory–TFTs, FBC, electrolytes, Ca²⁺, Mg²⁺, PO₄³⁻; add calcitonin & metanephrines for suspected medullary carcinoma.
Intra-operative Management
| Aspect | Best practice |
|---|---|
| Induction | Standard IV or inhalational; pre-oxygenate with HFNO > 40 l min⁻¹ to prolong safe apnoea time. |
| Intubation | Video-laryngoscopy is first-line. Awake fibre-optic reserved for severe compression, fixed obstruction or previous failure. Recent multicentre data show failed asleep intubation in mediastinal goitre is < 1 %. |
| Backup | Rigid bronchoscopy with jet ventilation; CPB/ECMO for invasive malignancy crossing carina. |
| Maintenance | TIVA or volatile. Avoid sustained neuromuscular block if continuous IONM used–give ≤ 0.3 mg kg⁻¹ cisatracurium after V1 mapping, then infuse remifentanil/propofol. |
| Haemodynamics | Esmolol 50–300 µg kg⁻¹ min⁻¹ blunts sympathetic surges; titrate fluids to avoid venous engorgement. |
| Position | Head-up 15°, neck neutral to slight extension; protect eyes and ulnar nerves. |
| Adjuncts | Dexamethasone 8 mg IV (anti-emetic, reduces airway oedema). Tranexamic acid 10 mg kg⁻¹ IV may reduce bleeding. |
| Extubation | Fully awake, head-up. Perform leak test; consider cuff-down fibre-optic inspection. If doubtful, leave ETT or exchange catheter and observe in HDU. |
Post-operative Concerns
- Neck haematoma (0.3–1 %)–any airway compromise → open wound immediately.
- Hypocalcaemia–check ionised Ca²⁺ at 6 h; symptomatic or Ca²⁺< 1.0 mmol l⁻¹ → 10 ml 10 % CaCl₂ IV.
- Recurrent laryngeal nerve (RLN) palsy–temporary 1–5 %, permanent < 1 %; higher with re-do surgery or malignancy.
- Tracheomalacia–high suspicion with long-standing goitre; may require staged extubation or tracheostomy.
Hyperthyroidism
Summary
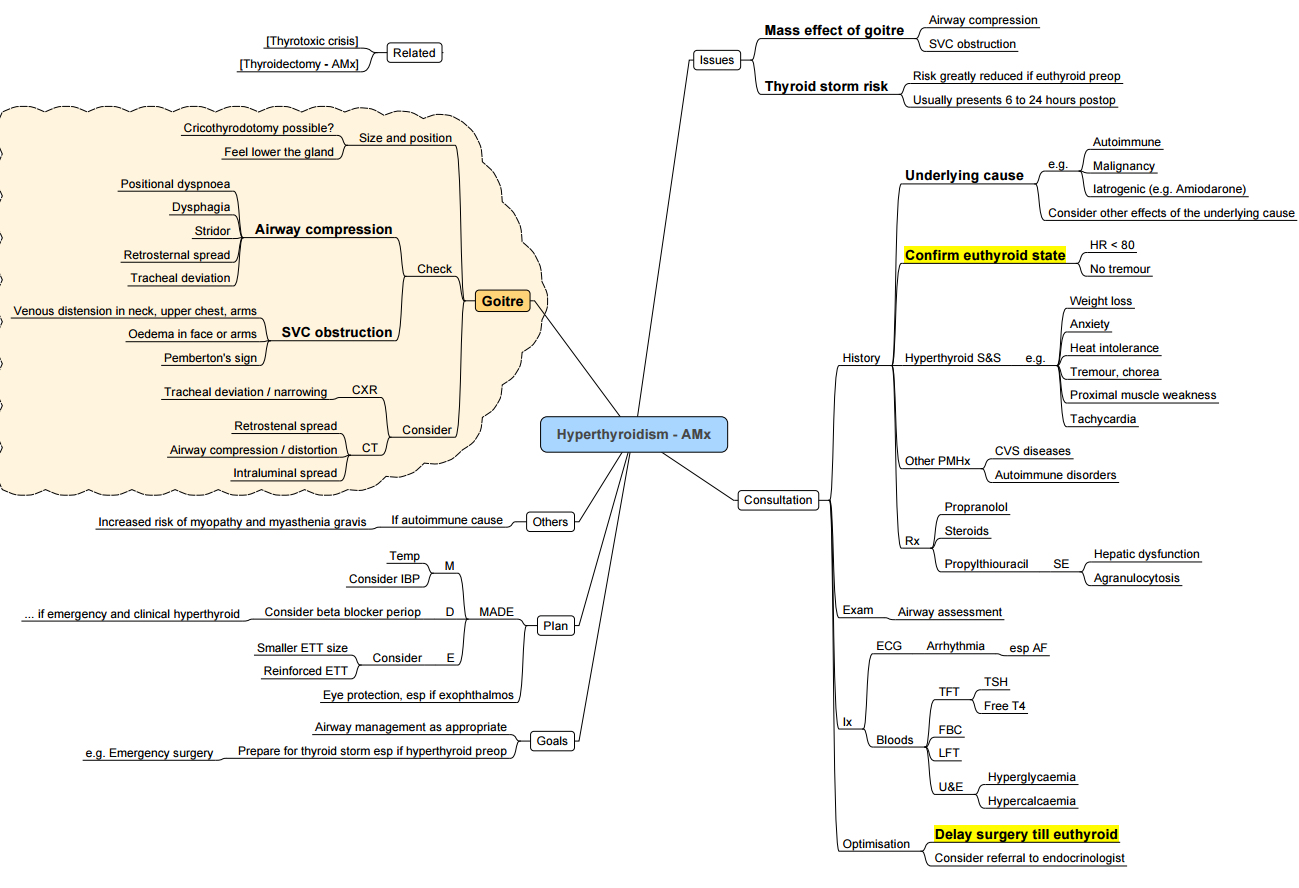
Pathogenesis and Clinical Findings
View or edit this diagram in Whimsical.
Causes
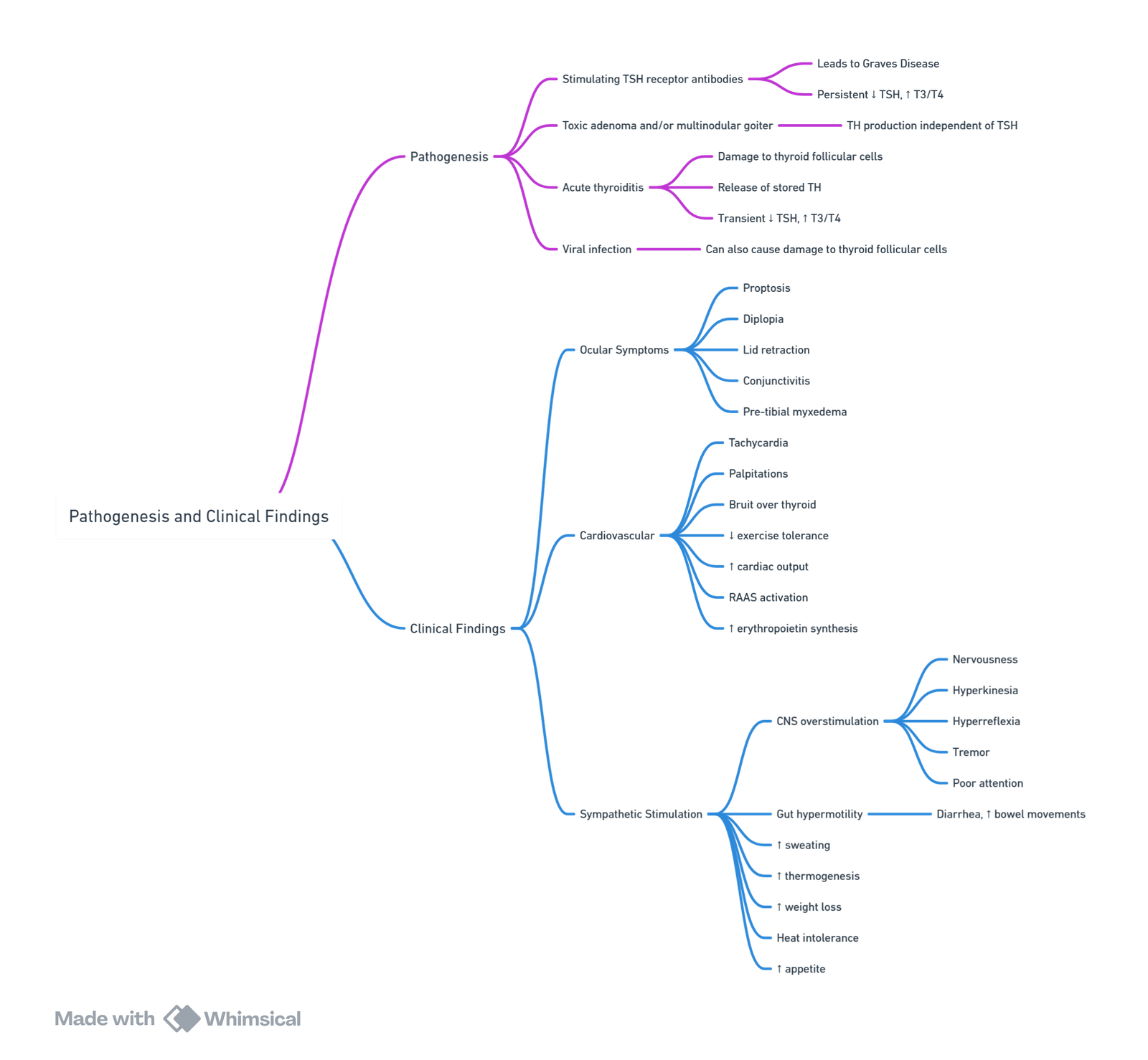
- Graves’ disease (autoimmune), toxic multinodular goitre, toxic adenoma, TSH-secreting pituitary adenoma, iodine-induced (amiodarone, contrast), gestational trophoblastic disease, excess thyroid hormone ingestion.
Diagnosis
- Suppressed TSH with elevated free T4 ± T3. T3-toxicosis (isolated high T3) is common in Graves’.
Optimising the Surgical Patient
- Timing of Surgery:
- Surgery is postponed until the patient is clinically and chemically euthyroid with normal T3 and T4 levels and no resting tachycardia.
- Elective Surgery: Defer if hyperthyroid (6-8 weeks). If urgent, manage with β-blockers, iodide, propylthiouracil (PTU) via nasogastric tube (NG), and glucocorticoids (dexamethasone).
| Drug | Typical dose | Comments |
|---|---|---|
| Carbimazole | 15–40 mg day⁻¹ PO | Pro-drug for methimazole; block TPO. |
| Propylthiouracil | 50–100 mg 8-hourly PO | Also blocks peripheral T4→T3; preferred in first-trimester pregnancy & thyroid storm. |
| Potassium iodide (Lugol’s 5 %) | 5 drops (≈ 50 mg) 8-hourly PO | Start ≥ 1 h after thionamide; blocks release (“Wolff–Chaikoff”). |
| Propranolol | 40 mg PO 6-hourly or 1 mg IV every 5 min | Controls HR, reduces T4→T3. |
| Hydrocortisone | 100 mg IV 8-hourly | Inhibits T4→T3; cover for adrenal insufficiency. |
- Radio-iodine (I-131) is contraindicated in pregnancy and uncontrolled thyrotoxicosis. Sub-total or total thyroidectomy is definitive when medical therapy fails or malignancy/goitre size dictates
Thyroid Storm (Thyrotoxic Crisis)
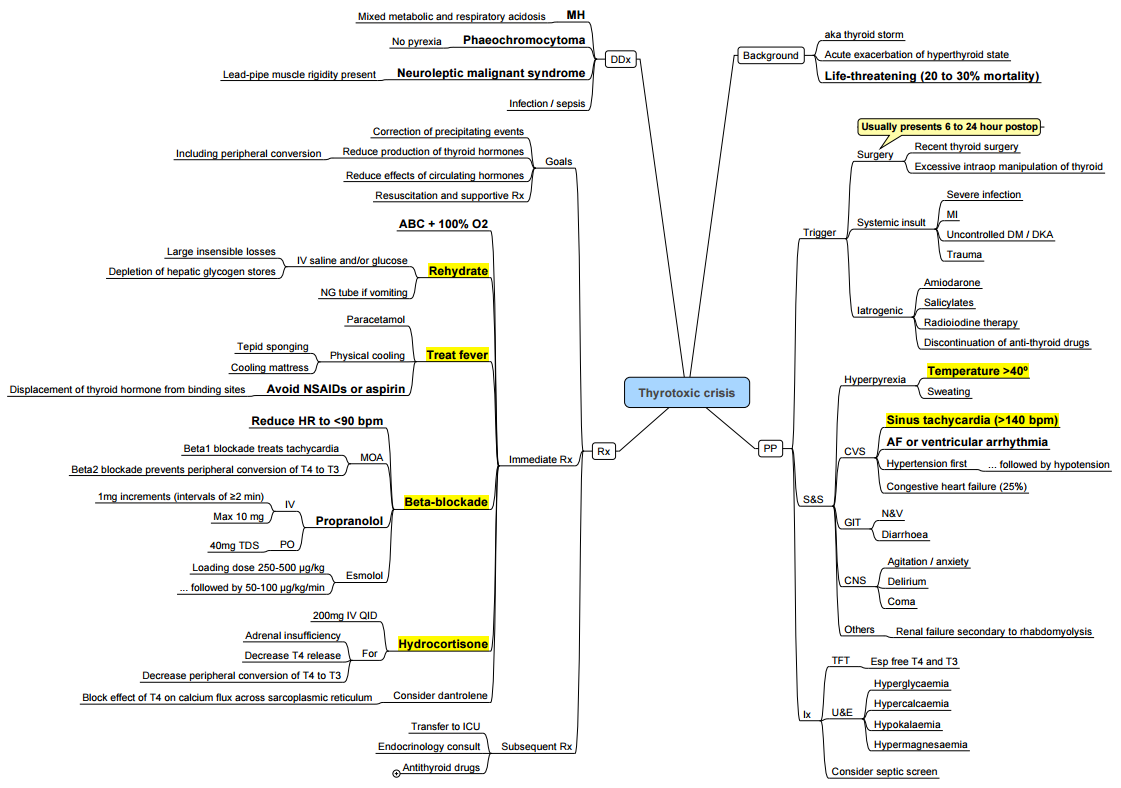
Recognition
- Hyperpyrexia (> 38.5 °C), tachyarrhythmia, CNS agitation or coma, heart failure, gastrointestinal upset. Burch-Wartofsky score > 45 suggests storm
Management (ABC-“5 B’s”)
- Block sympathetic–Esmolol 250–500 µg kg⁻¹ IV bolus then 50–100 µg kg⁻¹ min⁻¹.
- Block synthesis–Propylthiouracil 500–1000 mg PO/NG.
- Block release–Lugol’s solution 10 drops PO 1 h later.
- Block conversion–Hydrocortisone 100 mg IV 8-hourly.
- Supportive–Cool, monitor glucose & electrolytes, treat precipitant, admit ICU.
Complications of Thyroidectomy
| Immediate (0–24 h) | Early (24 h–2 w) | Late |
|---|---|---|
| Airway obstruction from haematoma | Temporary RLN palsy | Permanent RLN palsy |
| Laryngeal oedema/tracheomalacia | Hypocalcaemia/parathyroid stunning | Hypothyroidism |
| Vocal-cord dysfunction | Chyle leak (if thoracic duct injured) | Keloid, hypertrophic scar |
| Superior laryngeal nerve injury (voice pitch change) | Seroma, wound infection | Voice fatigue |
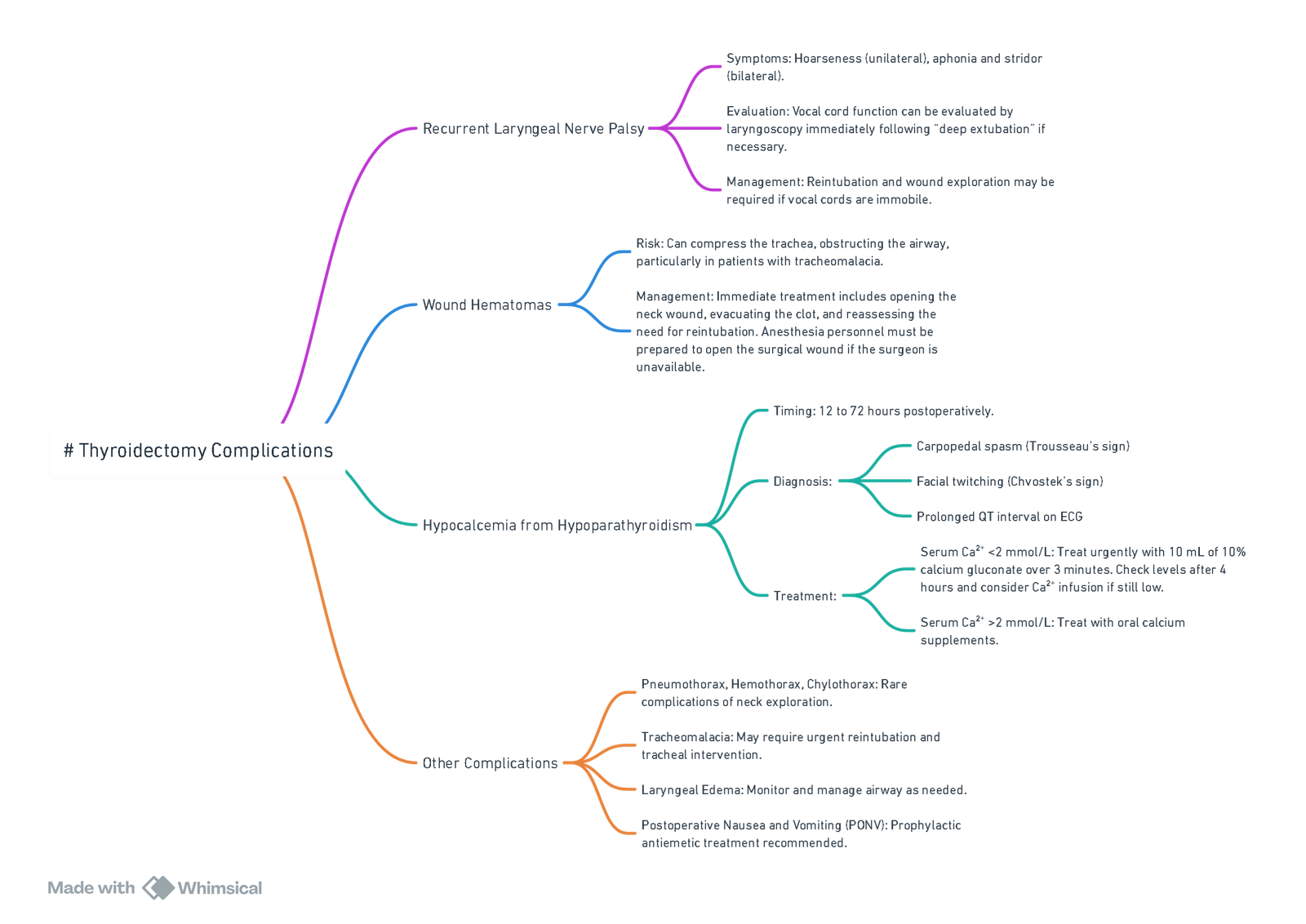
View or edit this diagram in Whimsical.
Hypothyroidism
- Hypothyroidism is common (female > male, peak incidence 40–60 yr) and ranges from sub-clinical disease to the life-threatening state of myxoedema coma.
- Thyroid hormone deficiency slows metabolism in every organ system, producing bradycardia, hypoventilation and impaired drug clearance–all highly relevant to anaesthesia.
- Elective surgery should be deferred until patients are clinically and biochemically euthyroid unless urgency outweighs risk.
- In emergencies, intravenous thyroid hormone plus stress-dose steroids restore cardiovascular stability within hours and permit safe anaesthesia.
Summary
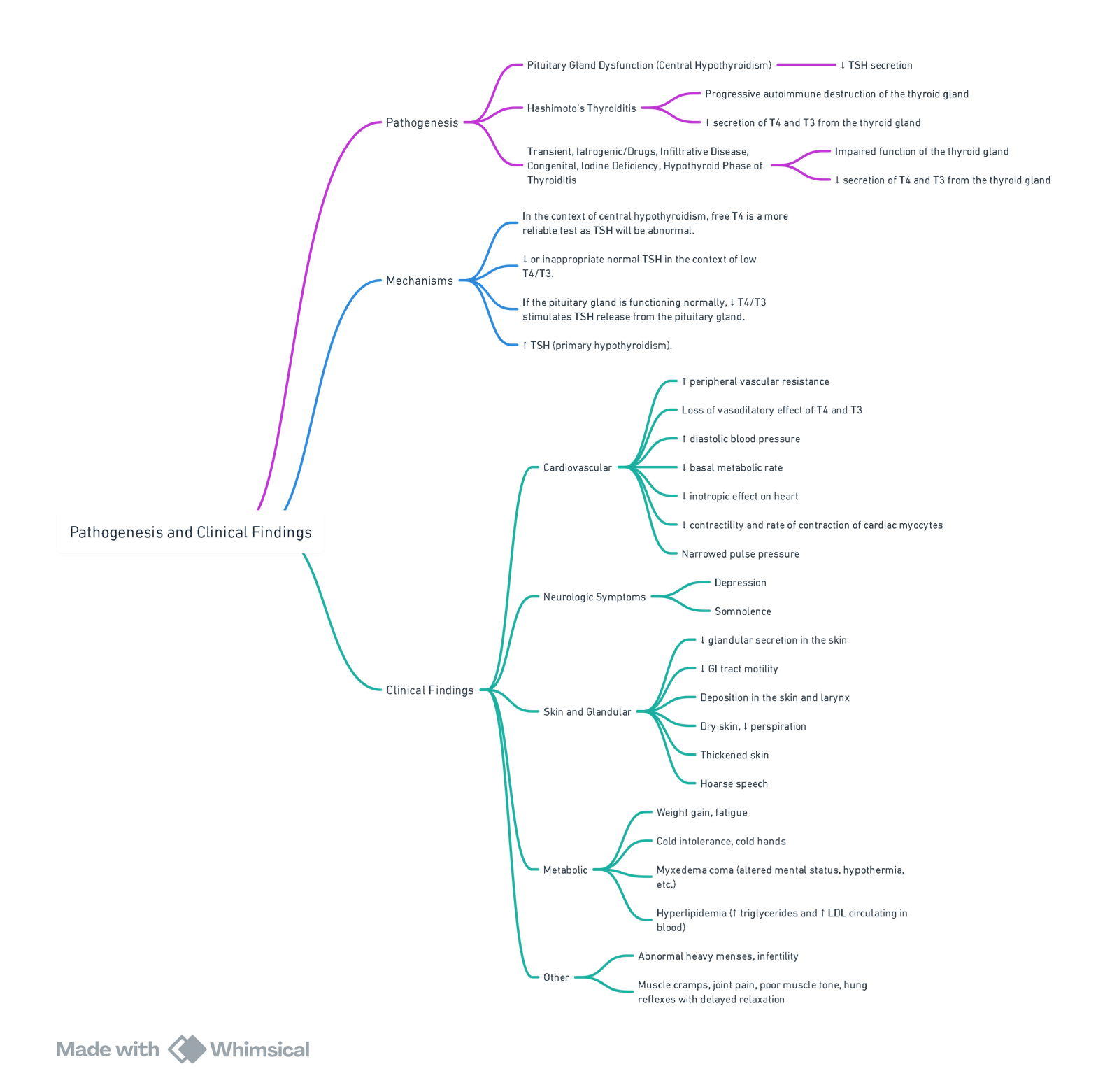
Pathogenesis and Causes
- Primary (≈ 95 %)—autoimmune (Hashimoto thyroiditis), thyroidectomy, radio-iodine, external neck irradiation, antithyroid drugs, iodine deficiency or excess, infiltrative disease, congenital biosynthetic defects.
- Secondary / Tertiary—hypothalamic-pituitary failure (tumour, surgery, trauma, infiltrative disorders).
- Drug-induced—lithium, amiodarone, interferon-α, tyrosine-kinase inhibitors.
- Neonatal—maternal iodine deficiency/endemic cretinism, dyshormonogenesis.
Severity Classification
| Severity | Typical biochemistry | Predominant clinical features | Peri-operative plan |
|---|---|---|---|
| Sub-clinical | TSH 4.5–10 mU l⁻¹, normal FT₄ | Often asymptomatic | Proceed with routine care |
| Overt (moderate) | TSH ≥ 10 mU l⁻¹ and/or low FT₄ | Lethargy, weight gain, bradycardia, effusions | Optimise with oral levothyroxine before elective surgery |
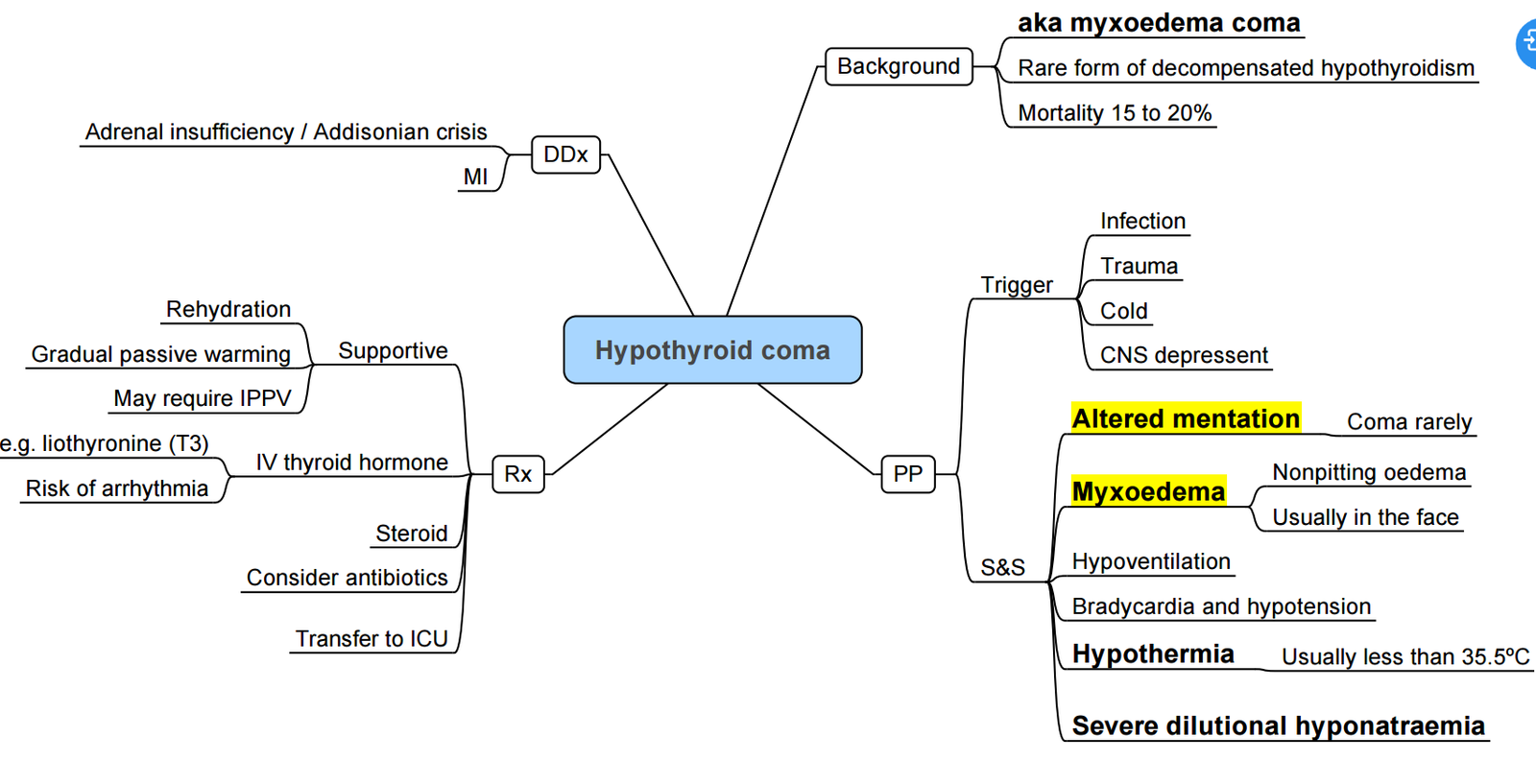
| Severe / Myxoedema | Marked ↑TSH (if primary) with very low or unmeasurable FT₄, hyponatraemia, hypoglycaemia | Hypothermia, hypotension, ventilatory failure, coma | ICU, IV thyroid hormone ± T₃, hydrocortisone; postpone surgery |
Clinical Manifestations
- General: cold intolerance, weight gain, dry skin, periorbital oedema, myxoedema facies.
- Cardiovascular: sinus bradycardia, ↓stroke volume & cardiac output, pericardial effusion, diastolic hypertension.
- Respiratory: hypoventilation, impaired response to hypoxia/hypercapnia, sleep-disordered breathing.
- Neuromuscular: fatigue, myalgia, delayed tendon reflexes.
- Gastro-intestinal: constipation, ileus, delayed gastric emptying.
- Haematological / Metabolic: normocytic anaemia, hyponatraemia, hypoglycaemia, hypercholesterolaemia.
Diagnosis
| Pattern | Interpretation |
|---|---|
| ↑TSH, ↓FT₄ | Primary hypothyroidism |
| Normal/low TSH, ↓FT₄ | Secondary/tertiary hypothyroidism |
| Normal TSH, low T₃ ± low T₄ | Non-thyroidal (euthyroid-sick) syndrome |
- T₃ falls late in primary disease and is unreliable for diagnosis.
Management
Long-term Replacement
- Levothyroxine (T₄): initial 1.6 µg kg⁻¹ day⁻¹ orally (lower in elderly or cardiac disease); adjust every 6–8 weeks to keep TSH 0.5–2.5 mU l⁻¹.
- Liothyronine (T₃) reserved for combination therapy trials or myxoedema coma.
Myxoedema Coma (medical emergency)
- Airway & ventilation; passive re-warming.
- IV levothyroxine 4 µg kg⁻¹ loading (200–400 µg), then 50–100 µg daily.
- IV liothyronine 5–20 µg followed by 2.5–10 µg 8to 12-hourly (omit or use low dose in elderly/ischaemic heart disease).
- Hydrocortisone 100 mg IV 8-hourly until adrenal insufficiency excluded
- Treat precipitant (sepsis, MI, stroke, trauma, surgery).
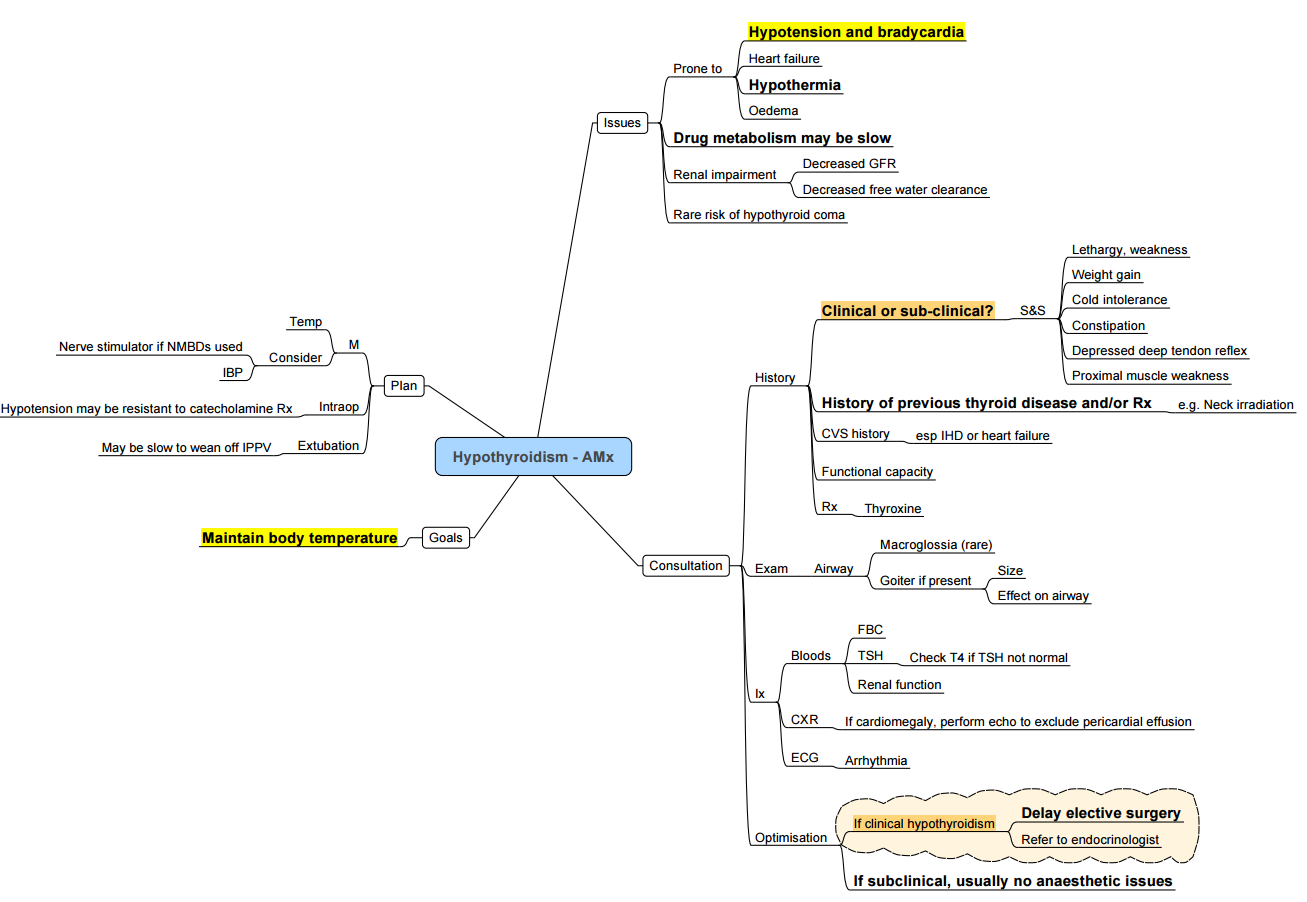
Anaesthesia for the Hypothyroid Patient
General Principles
- Mild–moderate disease: usually safe for surgery; continue oral levothyroxine on day of operation.
- Severe disease: delay elective surgery; if unavoidable, give IV T₄/T₃ and steroids as above.
System-by-system Considerations
| System | Implication | Management |
|---|---|---|
| Airway | Macroglossia, pharyngeal oedema, large goitre | Prepare for difficult mask & intubation; have smaller tubes & bougies ready |
| Cardiovascular | ↓CO, blunted baroreflex, pericardial effusion | Avoid hypotension; consider invasive arterial line ± TOE; use ephedrine or low-dose adrenaline rather than phenylephrine |
| Respiratory | Hypoventilation, OSA, pleural effusion | Controlled ventilation preferred; monitor capnography closely |
| Gastro-intestinal | Delayed gastric emptying | Rapid-sequence induction, pro-kinetic/metoclopramide |
| Haematology | Anaemia, factor VIII deficiency, platelet dysfunction | Correct coagulopathy; cell-saver if major blood loss expected |
| Temperature & metabolism | Hypothermia, hyponatraemia, hypoglycaemia | Active warming, warmed fluids, hourly glucose & electrolytes |
| Pharmacodynamics | Prolonged action of sedatives, NMBs, opioids | Reduce doses; use short-acting agents (propofol, remifentanil, atracurium); monitor neuromuscular blockade |
Induction & Maintenance
- Etomidate or ketamine maintain BP; avoid large thiopentone/propofol boluses.
- Volatile or TIVA acceptable; MAC unchanged.
- Titrate non-depolarising relaxants to twitch monitoring (delayed recovery likely).
Post-operative Care
- Extubate fully awake; observe in HDU if airway oedema, effusion or severe disease.
- Monitor temperature, electrolytes, glucose and TSH/FT₄.
- Continue usual levothyroxine early (enteral) or IV if NPO.
Complications
| Early | Late |
|---|---|
| Myxoedema coma, severe hypotension, ventilatory failure | Persistent bradycardia, pericardial effusion, delayed drug clearance |
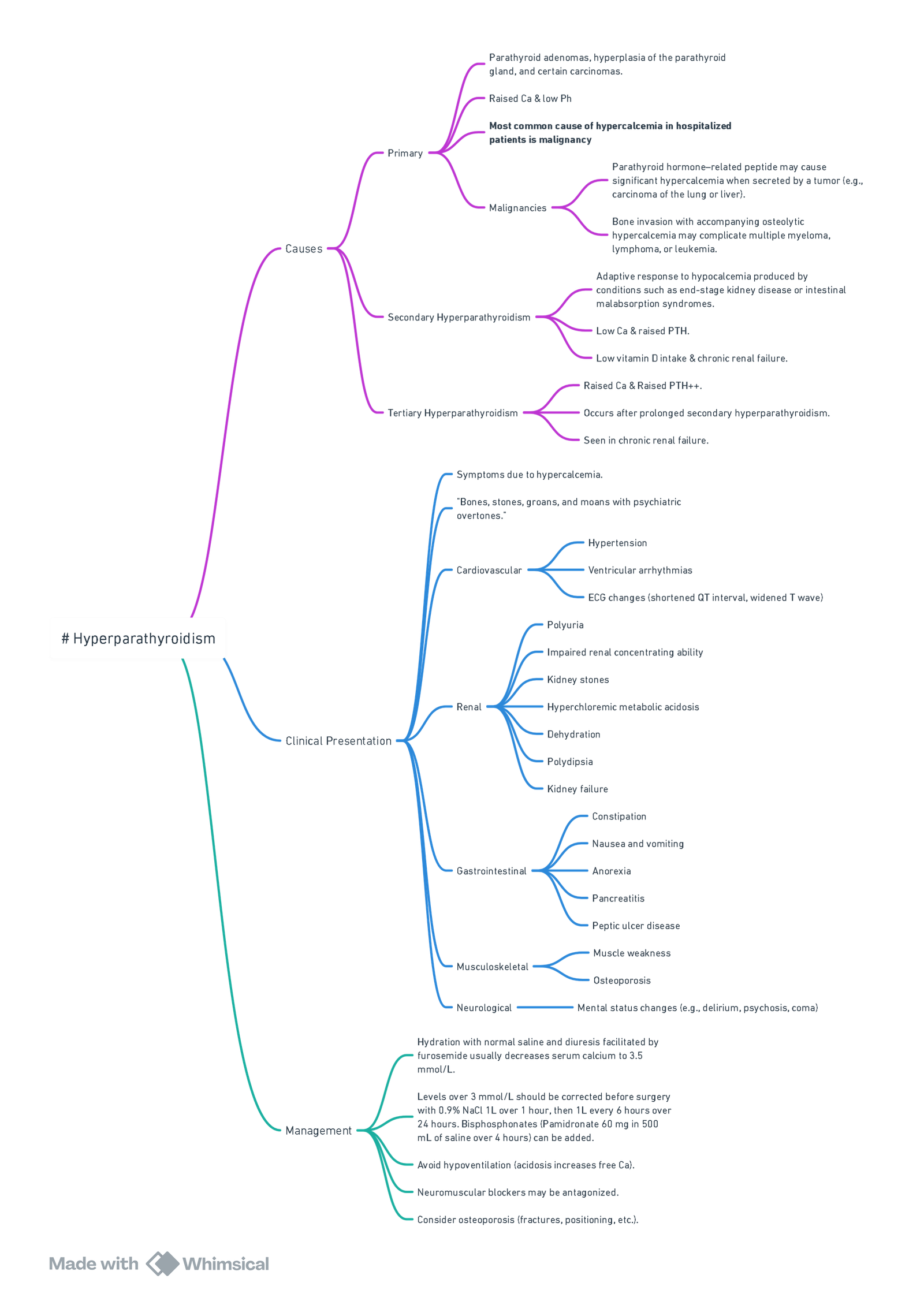
Parathyroid
Key Points
- Parathyroid hormone (PTH) is the principal regulator of extracellular ionised calcium; even minor changes trigger large shifts in neuromuscular excitability and cardiovascular function.
- Primary hyperparathyroidism (PHPT) is the commonest indication for parathyroidectomy and the only curative treatment. Minimally-invasive surgery guided by intra-operative PTH (ioPTH) sampling now achieves > 95 % cure with < 2 % recurrent laryngeal nerve (RLN) injury.
- Severe hypercalcaemia (Ca²⁺ > 3.0 mmol l⁻¹) increases peri-operative arrhythmia, gastrointestinal bleed and acute kidney injury; correct with volume resuscitation ± bisphosphonate or calcitonin before theatre.
- Hungry-bone syndrome (HBS) is the key post-operative threat; early recognition and aggressive calcium/activated-vitamin-D replacement reduce ICU admission and prolonged stay
Calcium-regulating Hormones
| Hormone | Bone | Kidney | Gastro-intestinal tract | Net effect on [Ca²⁺]ᵢ |
|---|---|---|---|---|
| PTH | ↑ Osteoclastic resorption | ↑ Ca²⁺ reabsorption, ↓ PO₄³⁻ & HCO₃⁻ reabsorption, ↑ 1α-hydroxylase ( → calcitriol) | ↑ Ca²⁺ absorption via calcitriol | ↑ |
| Calcitriol [1,25(OH)₂D₃] | Synergistic with PTH for bone turnover | Minor ↑ Ca²⁺ reabsorption | ↑↑ Ca²⁺ & PO₄³⁻ absorption | ↑ |
| Calcitonin | ↓ Osteoclastic resorption | ↓ Ca²⁺ & PO₄³⁻ reabsorption | Minimal | ↓ |
Fibroblast-growth-factor-23 (FGF-23) from osteocytes also lowers PO₄³⁻ and vitamin-D levels but has little acute peri-operative impact.
Primary Hyperparathyroidism
| Typical biochemistry | Common manifestations (remember “STONES, BONES, GROANS, MOANS”) |
|---|---|
| ↑ Serum Ca²⁺ (> 2.6 mmol l⁻¹) with inappropriately high PTH | Nephrolithiasis/nephrocalcinosis, osteoporosis or fragility fractures, dyspepsia/peptic ulcer, depression, muscle weakness, shortened QT |
- Indications for surgery (AAES 2023)–symptomatic disease; serum Ca²⁺ > 0.25 mmol l⁻¹ above ULN; T-score < −2.5 or vertebral fracture; eGFR < 60 ml min⁻¹ 1.73 m⁻²; age < 50 y.
Anaesthesia for Parathyroidectomy
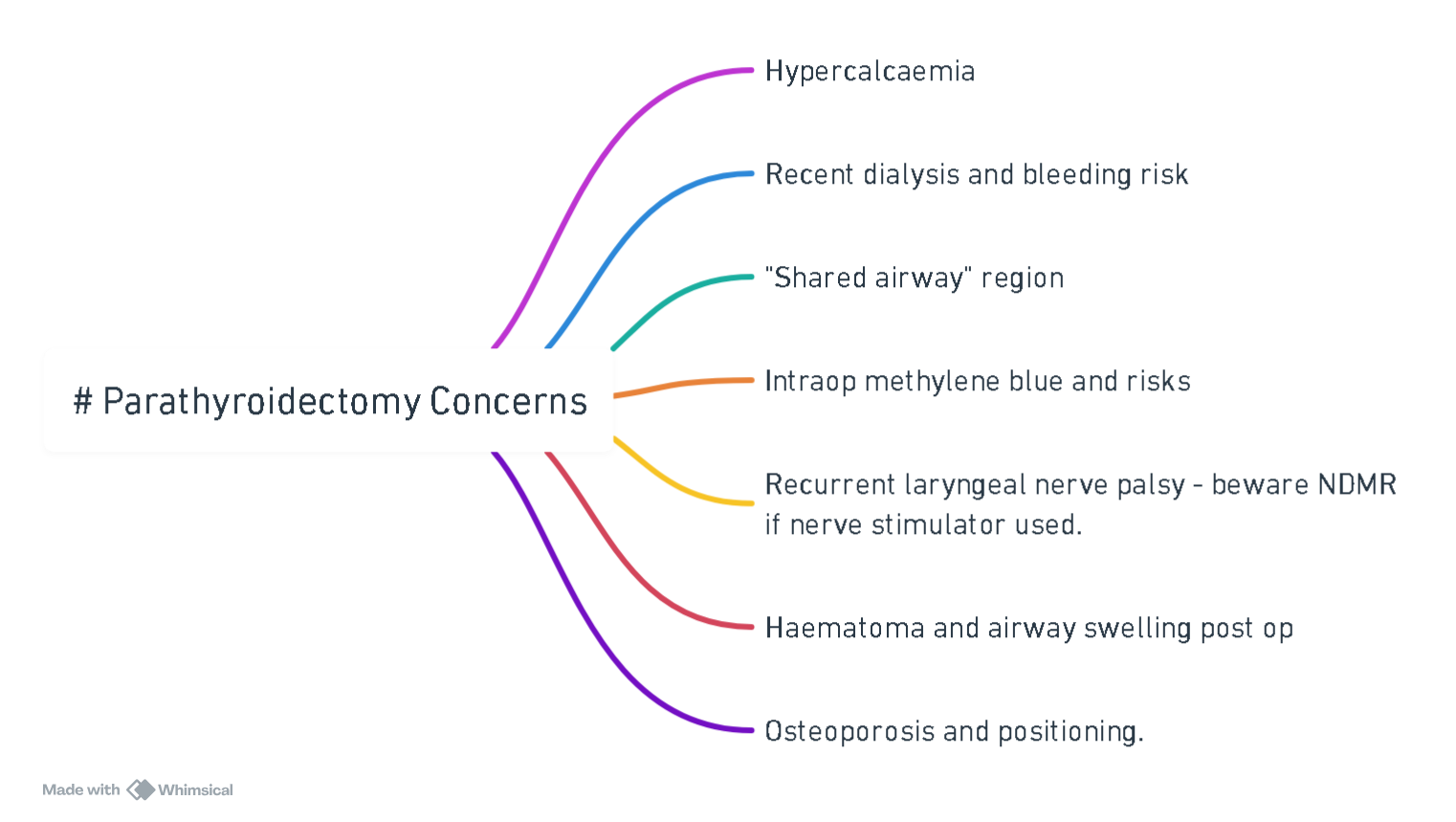
Pre-operative
- Optimise calcium–hydrate with 0.9 % saline (100–200 ml h⁻¹), add loop diuretic once euvolaemic; give IV bisphosphonate (zoledronic acid 4 mg) or calcitonin 4 IU kg⁻¹ if Ca²⁺ ≥ 3 mmol l⁻¹.
- Evaluate end-organ impact–ECG (short QT, AV block, VT), renal USS, DEXA; correct hypokalaemia, hypomagnesaemia.
- Drug review–discontinue thiazides, lithium, digoxin; continue cinacalcet until morning of surgery.
Intra-operative
| Item | Recommendation |
|---|---|
| Anaesthesia | Balanced GA (± superficial cervical plexus block). Total IV anaesthesia facilitates ioPTH timing. |
| Airway | Standard ETT; video-laryngoscopy preferred for re-do surgery. Preserve RLN with intermittent or continuous IONM; avoid/antagonise NMB after first twitch mapping. |
| Fluids | Warmed crystalloids 2–3 ml kg⁻¹ h⁻¹; avoid lactate (spurious Ca²⁺ rise). |
| Neuromuscular blockade | Response unpredictable in chronic hyperCa²⁺; titrate to TOF. |
| Monitoring | Standard + arterial line if Ca²⁺ > 3 mmol l⁻¹, significant cardiac disease or revision surgery. |
Post-operative
- Hypocalcaemia / HBS
- Risk factors: pre-op Ca²⁺ > 2.85 mmol l⁻¹, PTH > 30 pmol l⁻¹, alkaline-phosphatase > 2× ULN, vitamin-D deficiency, renal failure.
- Monitor ionised Ca²⁺ 4-hourly for 24 h.
- Treat symptomatic or Ca²⁺ < 1.0 mmol l⁻¹ with 10 ml 10 % CaCl₂ IV over 10 min → continuous infusion 0.5 mg kg⁻¹ h⁻¹; add calcitriol 0.5–1 µg day⁻¹.
- Airway–RLN palsy (1 %) presents with stridor or hoarse voice; assess before extubation.
- Analgesia–paracetamol + NSAID (if renal function allows) + low-dose opioid; avoid oversedation in hyperCa²⁺-related CNS depression.
Secondary & Tertiary Hyperparathyroidism (renal failure)
- Often require subtotal (3½-gland) parathyroidectomy; keep dialysis Ca²⁺ bath ≤ 1.25 mmol l⁻¹ pre-op.
- Post-op HBS incidence up to 45 %; commence prophylactic oral calcium carbonate 1 g QID + calcitriol 0.5 µg immediately after surgery.
Complications of Parathyroidectomy
| Early (hours–days) | Late |
|---|---|
| Hypocalcaemia / HBS, RLN palsy, cervical haematoma, neck seroma | Persistent/recurrent PHPT (2–5 %), chronic voice fatigue, keloid |
Adrenal Gland
Summary
Key Points
- The adrenal cortex (zona Glomerulosa → aldosterone, Fasciculata → cortisol, Reticularis → androgens) and medulla (catecholamines) together coordinate cardiovascular stability, volume status and stress responses.
- Primary hyperaldosteronism is now recognised in up to 10 % of hypertensive patients and is cured by unilateral laparoscopic adrenalectomy; pre-operative optimisation of K⁺ and blood pressure is essential.
- Cushing’s syndrome carries a five-fold higher peri-operative thrombo-embolic risk and demands meticulous glucose, electrolyte and positioning precautions.
- Adrenal insufficiency is rare but lethal; 100 mg hydrocortisone IV at the first suspicion of crisis saves lives.
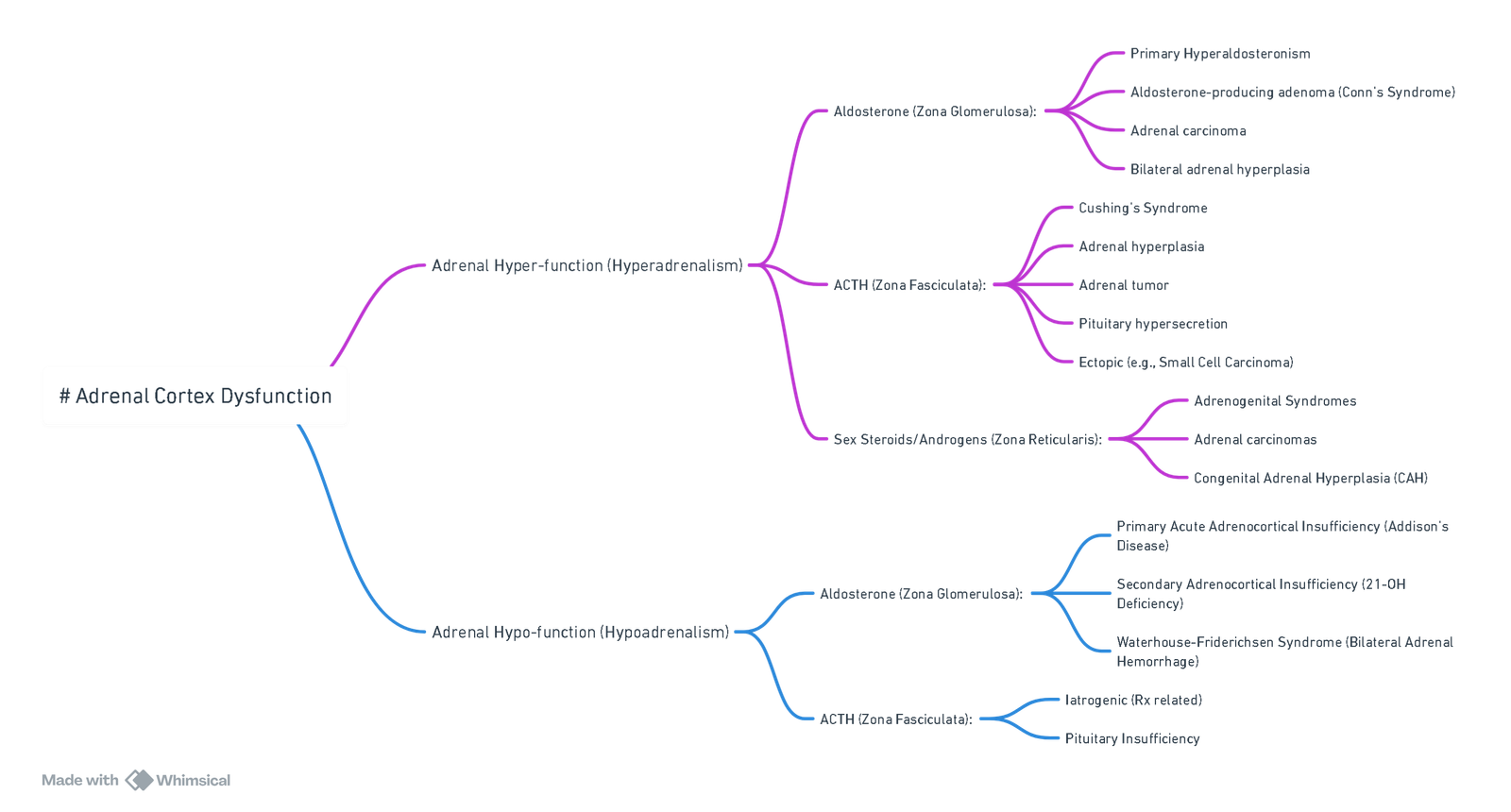
Physiology
Cortical Hormones
| Zone | Hormone | Principal actions | Key regulators | Notes |
|---|---|---|---|---|
| Glomerulosa | Aldosterone | ↑Na⁺ & H₂O reabsorption, ↑K⁺ & H⁺ excretion | Renin-angiotensin system, K⁺ | Acts via mineralocorticoid receptor in distal nephron |
| Fasciculata | Cortisol | Gluconeogenesis, anti-inflammatory, potentiates catecholamines | ACTH, diurnal rhythm, stress | 90 % protein-bound; peaks 08 h |
| Reticularis | DHEA, androstenedione | Pubic/axillary hair, libido | ACTH | Minor in adult males |
| Medulla | Epinephrine (80 %), norepinephrine (20 %) | Fight-or-flight response | Sympathetic pre-ganglionic Ach | Cortisol from cortex enhances PNMT activity |
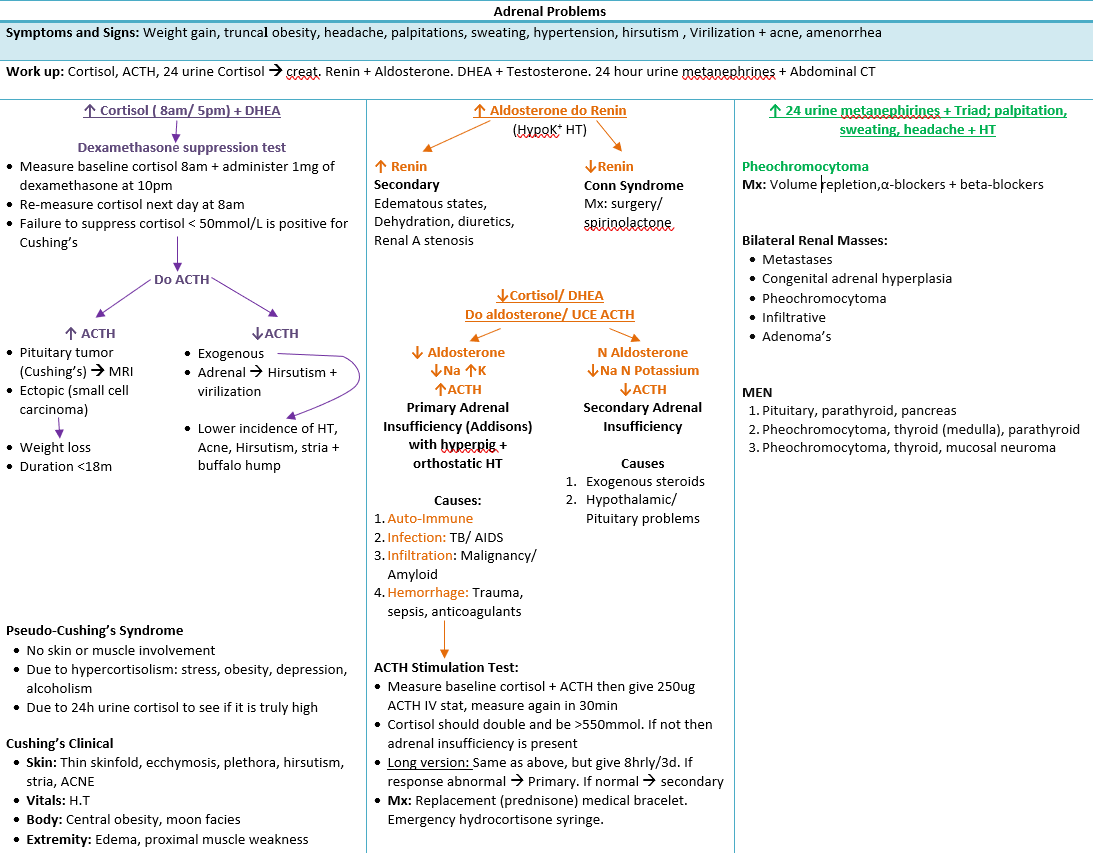
Disorders of Cortical Function
| Disorder | Hormones | Typical biochemistry | Salient features |
|---|---|---|---|
| Primary hyperaldosteronism (Conn) | ↑ Aldosterone, ↓ Renin | ARR > 20, PAC > 10 ng dl⁻¹ | Resistant hypertension, hypokalaemia, metabolic alkalosis |
| Secondary hyperaldosteronism | ↑ Aldosterone, ↑ Renin | Renovascular, CHF, cirrhosis | Volume overload signs |
| Cushing’s syndrome | ↑ Cortisol ± ACTH | Loss of diurnal variation, failure to suppress with dexamethasone | Central obesity, diabetes, myopathy, skin fragility, OSA |
| Primary adrenal insufficiency (Addison) | ↓ Cortisol & aldosterone, ↑ ACTH | Hyponatraemia, hyperkalaemia, eosinophilia | Hyperpigmentation, postural hypotension |
| Secondary adrenal insufficiency | ↓ Cortisol, normal aldosterone | Low/normal ACTH | Pituitary disease, chronic steroid therapy |
Primary hyperaldosteronism–anaesthetic Implications
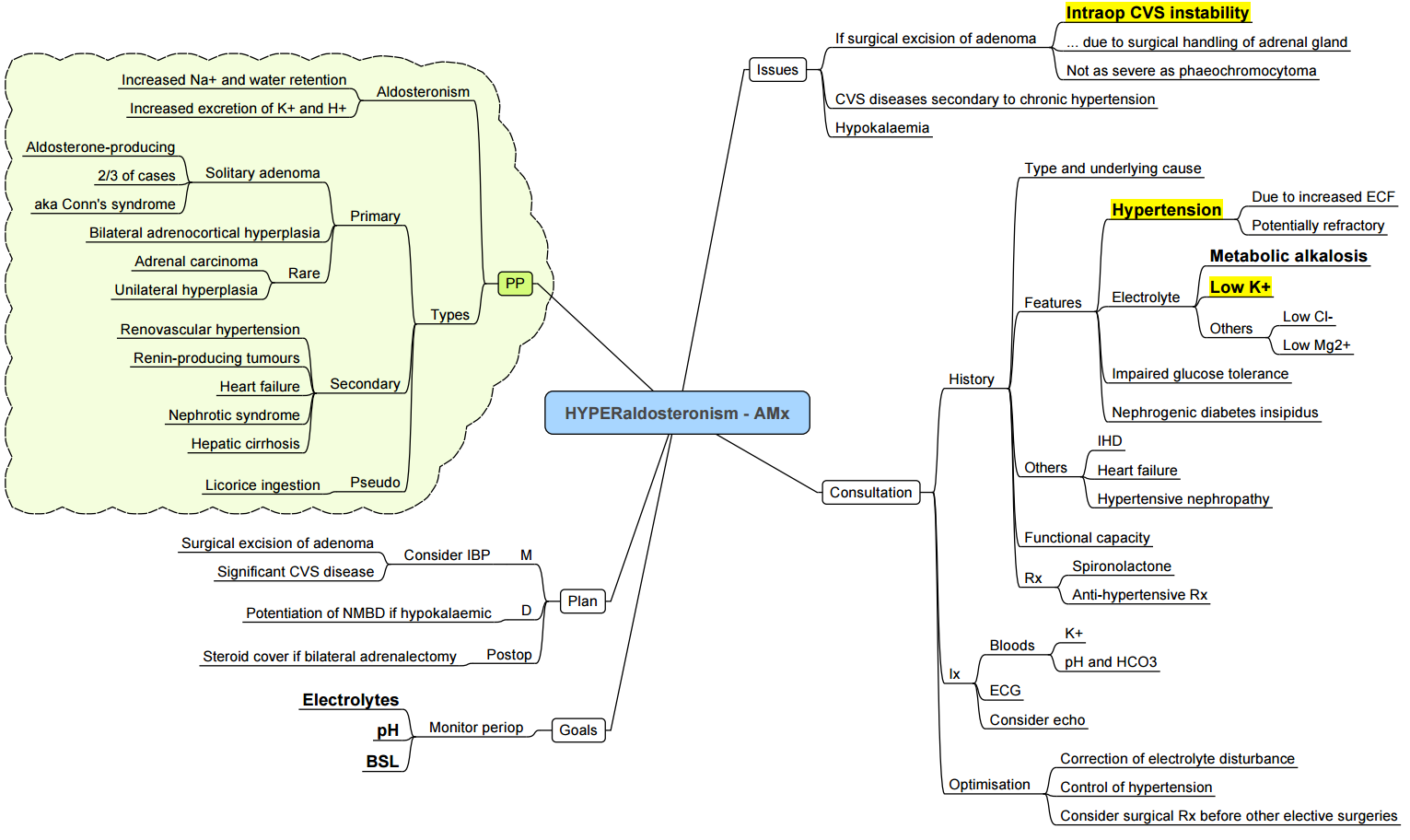
Pre-operative
- Normalise K⁺ > 3.5 mmol l⁻¹ with oral/IV supplements and mineralocorticoid receptor antagonists (spironolactone 25–100 mg day⁻¹).
- Control BP with calcium-channel blockers or MR antagonists; avoid ACE-I/ARB withdrawal if well-tolerated.
- Screen for obstructive sleep apnoea (OSA) and metabolic alkalosis (ABG).
Intra-operative
| Consideration | Strategy |
|---|---|
| Haemodynamics | Expect labile hypertension during adrenal manipulation; treat with short-acting vasodilator (phentolamine 1 mg IV or clevidipine). |
| Electrolytes | Check arterial K⁺ after induction and at closure; treat promptly. |
| Neuromuscular blockade | Chronic hypokalaemia may prolong non-depolarising block–titrate to TOF. |
| Acid–base | Avoid hyperventilation and large bicarbonate loads (worsen alkalosis). |
Post-operative
- Monitor K⁺ 6-hourly for 24 h–rebound hyperkalaemia can occur once aldosterone falls
- Most patients are normotensive within weeks; review antihypertensives at discharge.
Cushing’s syndrome–anaesthetic Considerations
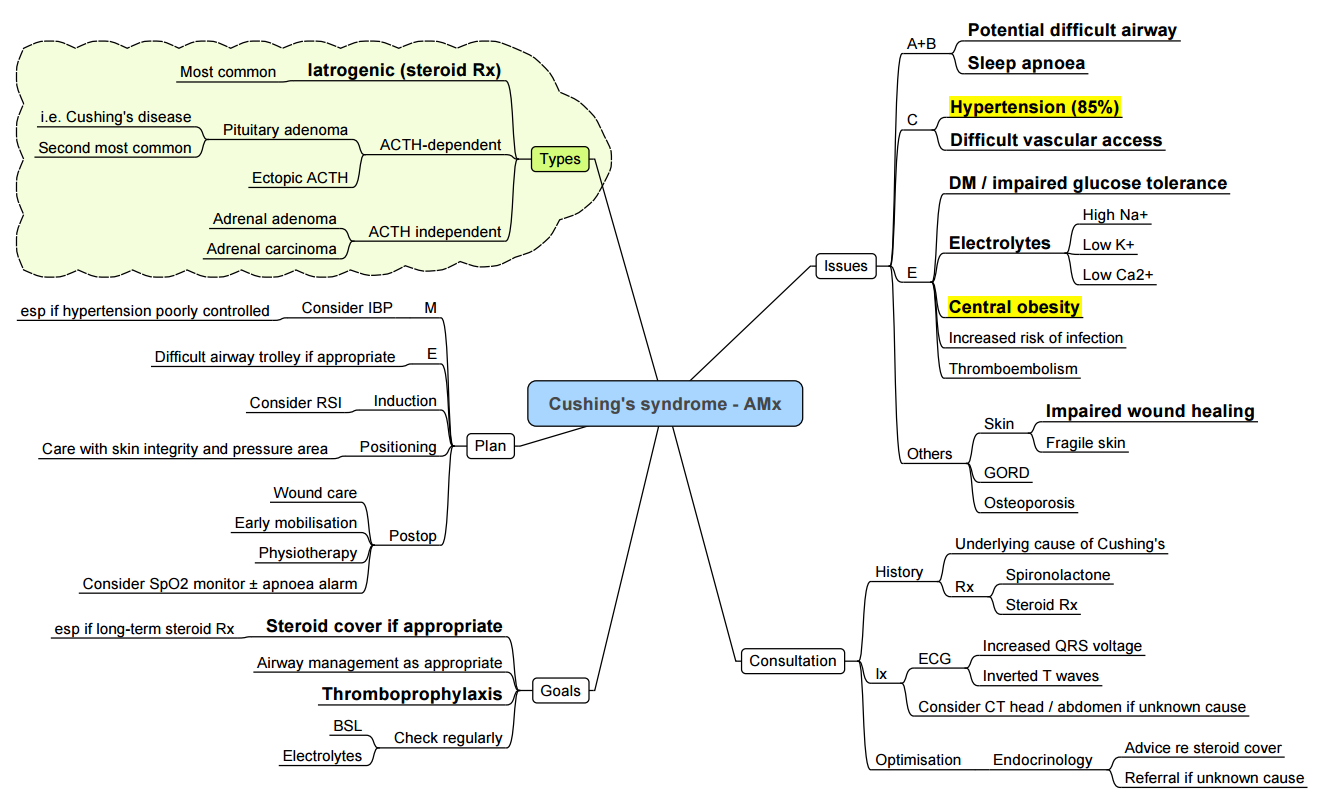
| System | Issues | Management |
|---|---|---|
| Airway & respiratory | Truncal obesity, buffalo hump, proximal myopathy, OSA | Ramped position, videolaryngoscope, CPAP pre-/post-op |
| Cardiovascular | Hypertension, LVH, accelerated CAD, hypercoagulability | Invasive BP, vigilant thromboprophylaxis, avoid fluid overload |
| Metabolic | Hyperglycaemia, hypokalaemia | Hourly capillary glucose, insulin infusion; replace K⁺ |
| Skin & MSK | Thin skin, osteoporosis | Careful positioning, generous padding, avoid excessive neck extension |
| Infection risk | Immunosuppression, poor wound healing | Strict asepsis, prophylactic antibiotics |
- Steroid cover: After adrenalectomy give hydrocortisone 100 mg IV at skin incision, then 50 mg IV 6-hourly × 24 h, tapering to physiological replacement.
Adrenal Insufficiency
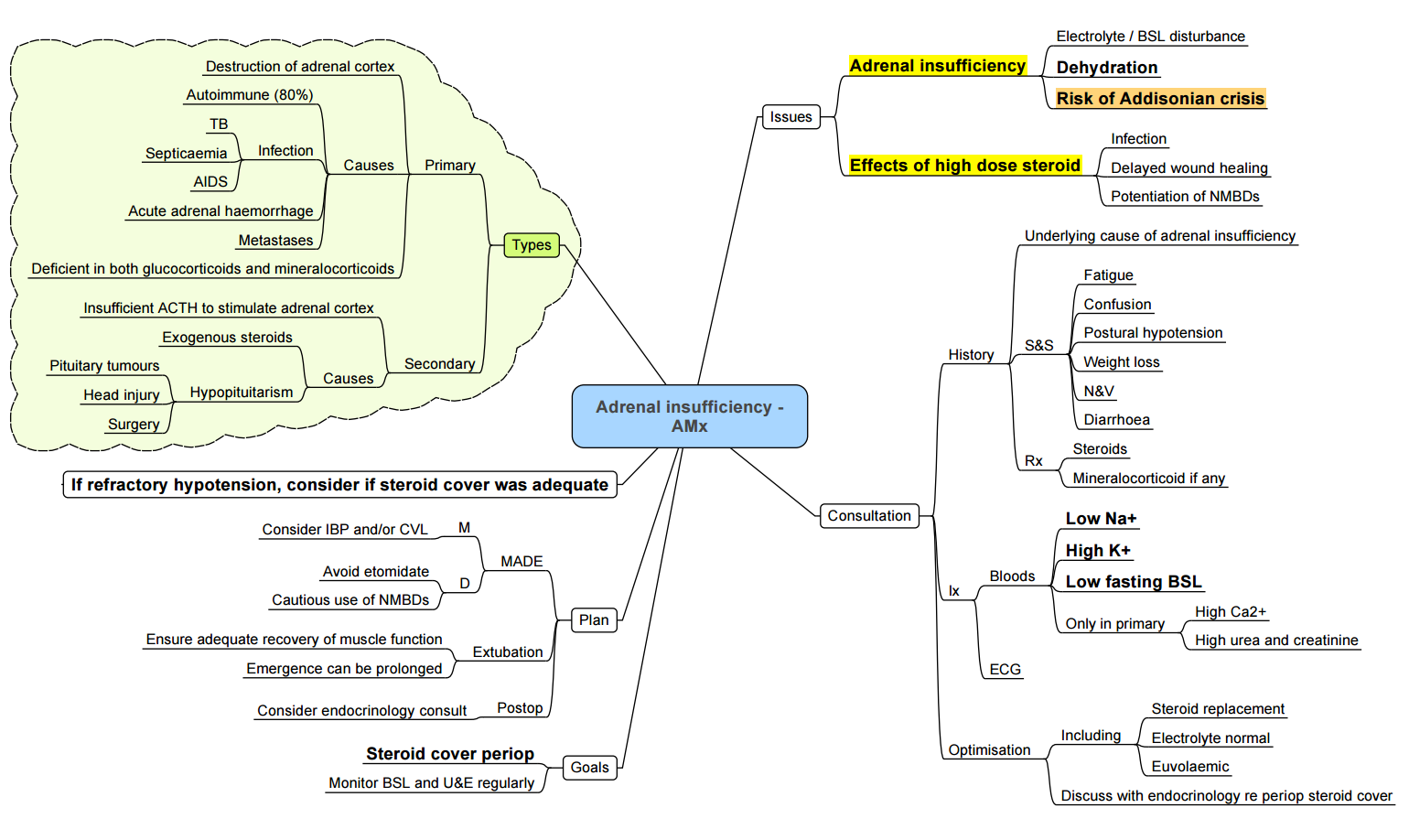
Addisonian (adrenal) Crisis
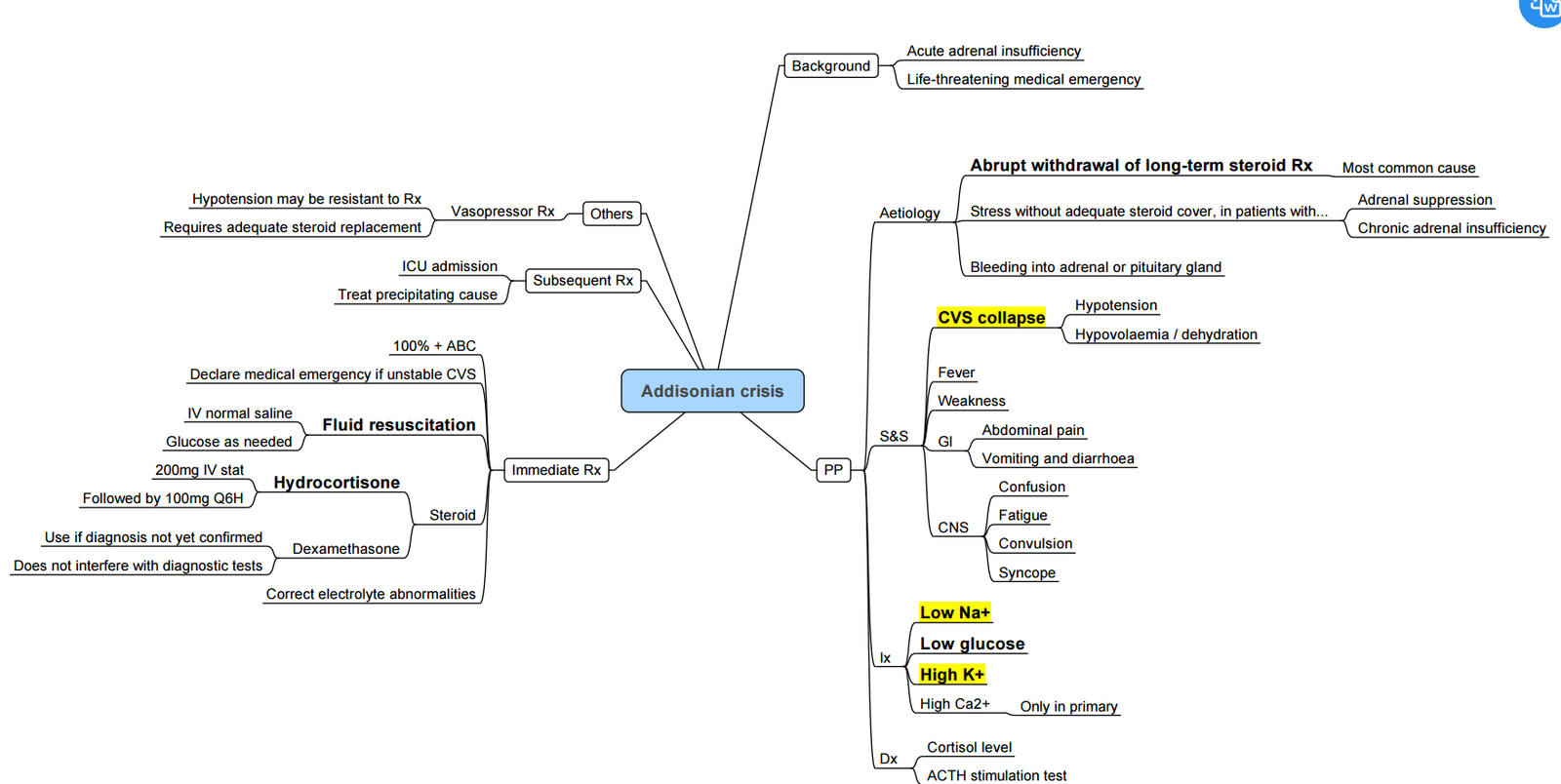
- Triggers: surgery, sepsis, trauma, etomidate infusion.
- Treat immediately (do not await labs): Hydrocortisone 100 mg IV, 1 l 0.9 % saline over 1 h, then 200 mg hydrocortisone / 24 h by infusion or 50 mg IV 6-hourly.
- Monitor Na⁺, K⁺, glucose; correct hypoglycaemia and hyperkalaemia.
Peri-operative Steroid Supplementation (UK 2020 guidance)
| Surgical stress | Examples | Additional hydrocortisone (above baseline) |
|---|---|---|
| Minor (e.g. ingrown toenail, cataract) | Local / MAC, < 30 min | None |
| Moderate (lap chole, hip replacement) | GA or regional, 1–2 h | 50 mg IV at induction then 25 mg 8-hourly × 24 h |
| Major (cardiac, bowel resection) | GA > 2 h, major blood loss | 100 mg IV at induction, then 200 mg / 24 h (infusion or 50 mg 6-hourly) until eating |
- Patients on > 5 mg prednisolone equivalent for > 2 weeks in the past year should be assessed for HPA suppression and receive cover per table above
Catecholamine Excess: Phaeochromocytoma & Paraganglioma (PPGL)
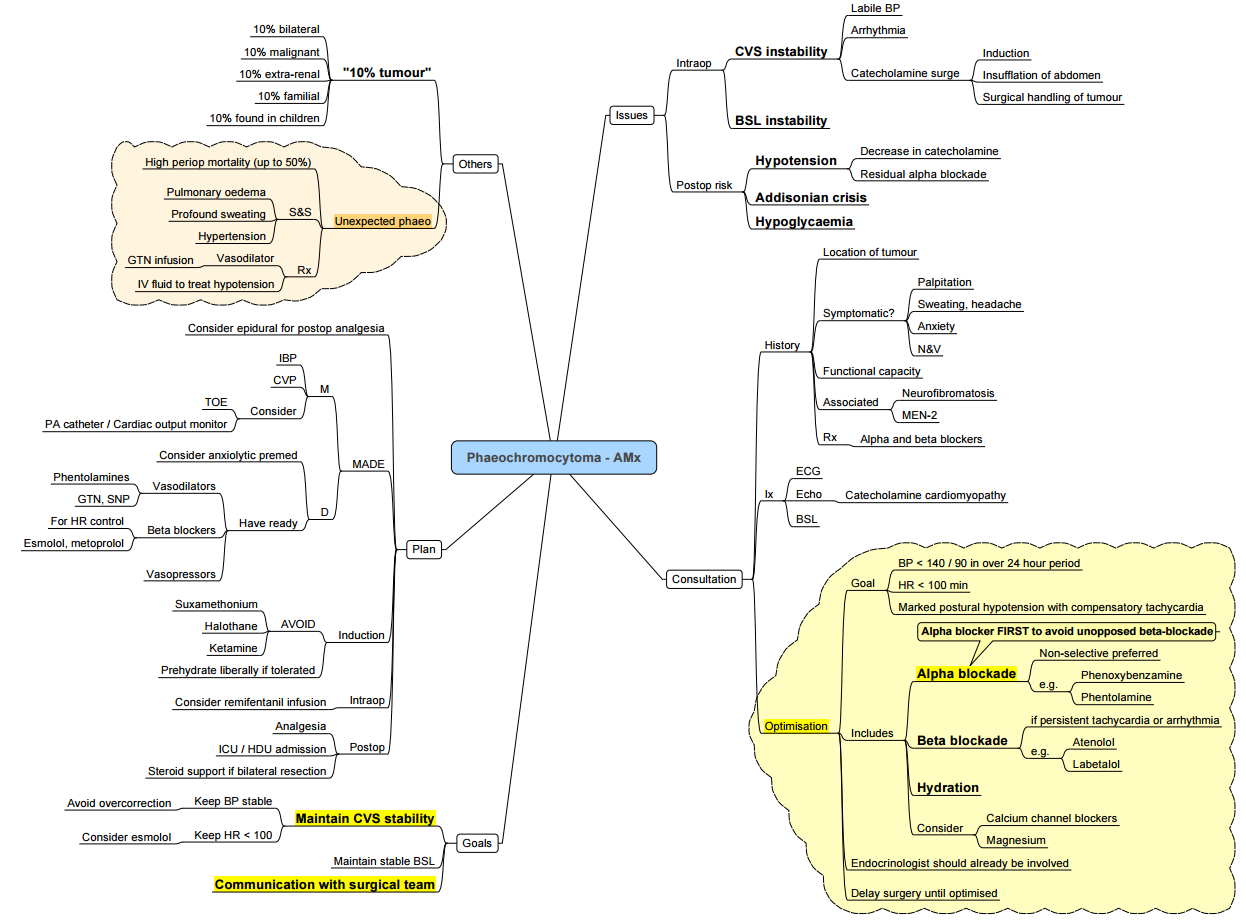
Key Points
- Catecholamine-secreting tumours of chromaffin tissue may arise in the adrenal medulla (phaeochromocytoma, PCC) or extra-adrenal paraganglia (paraganglioma, PGL).
- Classic triad: paroxysmal headache, sweating and palpitations against a background of sustained or episodic hypertension.
- Every patient with an adrenal incidentaloma or resistant hypertension must be screened–unsuspected cases carry a peri-operative mortality up to 50 %.
- Safe surgery hinges on meticulous α-blockade, volume expansion, and intra-operative haemodynamic control.
- Long-term cure rates exceed 90 %, but all patients require lifelong biochemical follow-up for recurrence or metastatic disease.
Pathogenesis & Genetics
- ~40 % of PPGLs are hereditary; common syndromes: MEN-2, Von Hippel-Lindau, Neurofibromatosis-1, SDHx mutations.
- Genetic testing is recommended for all patients (guideline expert consensus 2023).
Clinical Presentation
| Catecholamine effect | Manifestations |
|---|---|
| α-adrenergic | Severe hypertension, pallor, peripheral vasoconstriction, cardiomyopathy, hyperglycaemia |
| β-adrenergic | Tachyarrhythmias, tremor, anxiety, heat intolerance |
| Metabolic | Weight loss, impaired glucose tolerance |
| Paroxysms | Triggered by stress, induction, positioning, tumour manipulation |
Diagnosis
- Biochemistry
- Plasma free metanephrines (supine, cannulated)–highest sensitivity.
- 24 h urinary fractionated metanephrines/catecholamines if plasma equivocal.
- Localisation
- CT/MRI adrenals → 98 % detection.
- Functional imaging (⁶⁸Ga-DOTATATE PET/CT or ¹²³I-MIBG) for extra-adrenal, multifocal or metastatic disease.
- Pre-operative tests
- ECG ± echocardiography (LV hypertrophy, cardiomyopathy).
- Full blood count, U&E, glucose
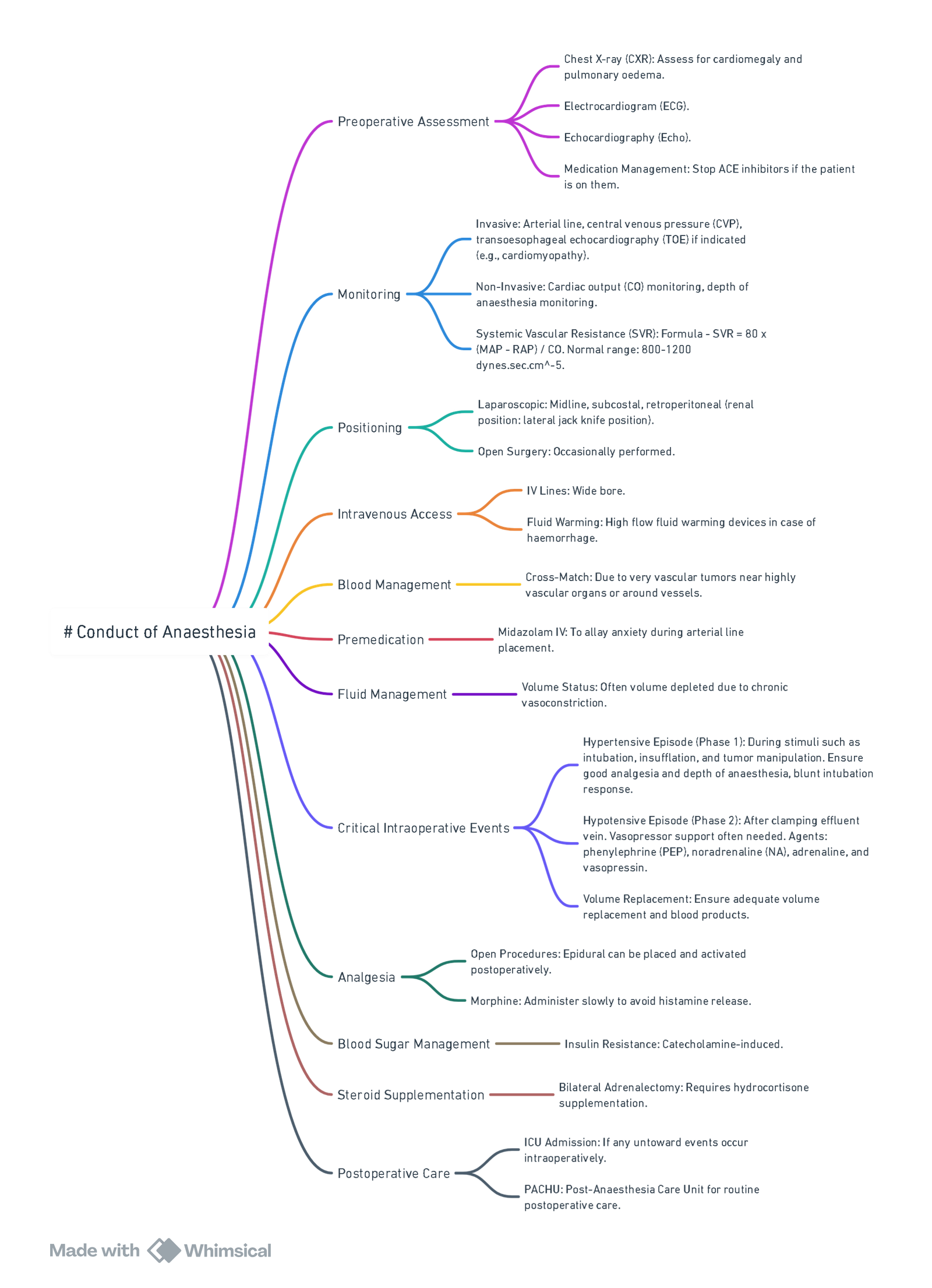
Pre-operative Optimisation
| Target | Measure |
|---|---|
| α-blockade (10–14 d) | Phenoxybenzamine 10 mg BD (↑ every 2 d) or selective α₁ blocker (doxazosin 2 mg → 8–16 mg). Aim seated BP < 130/80 mmHg, standing SBP > 90 mmHg, HR 60–80 bpm with mild orthostatic hypotension. |
| Volume expansion | High-sodium diet (≥ 5 g Na⁺ day⁻¹) + 2–3 l day⁻¹ fluid from day 5 to restore plasma volume (haematocrit ↓ ≤ 5 %). |
| β-blockade | Add propranolol or bisoprolol only after adequate α-blockade for tachycardia/arrhythmia control. |
| Adjuncts | Nifedipine or diltiazem for persistent hypertension; metyrosine (α-methyl-p-tyrosine) for metastatic or refractory disease. |
Anaesthesia for PPGL Resection
Monitoring & Access
- Arterial line before induction, ± large-bore venous sheath, urinary catheter.
- Consider central line and trans-oesophageal echocardiography (TOE) in large tumours or poor LV function.
Induction & Maintenance
| Phase | Suggested agents | Rationale |
|---|---|---|
| Induction | Propofol / Etomidate + opioid (fentanyl/remifentanil) + rocuronium | Attenuate sympathetic response; avoid histamine release & tachycardia |
| Maintenance | TIVA or sevoflurane ± remifentanil; BIS 40–60 | Permit rapid haemodynamic titration |
| Regional | Thoracic epidural (open cases) for analgesia; will not blunt catecholamine surges |
Haemodynamic Control
| Event | First-line drug (bolus or infusion) |
|---|---|
| Hypertension/tachycardia (manipulation) | Sodium nitroprusside 0.5–5 µg kg⁻¹ min⁻¹, phentolamine 30–100 µg kg⁻¹ bolus, nicardipine 2 mg bolus then 2 mg h⁻¹, magnesium sulphate 30 mg kg⁻¹ load → 1–2 g h⁻¹ |
| Tachyarrhythmia | Esmolol 0.5 mg kg⁻¹ → 50–200 µg kg⁻¹ min⁻¹ |
| Post-ligation hypotension | Volume 10–20 ml kg⁻¹ crystalloid, then norepinephrine 0.05–0.3 µg kg⁻¹ min⁻¹ ± vasopressin 0.4 U h⁻¹ |
- Avoid: ketamine, pancuronium, atropine, ephedrine, histamine-releasing opioids/muscle relaxants, metoclopramide.
Metabolic Watchpoints
- Hourly glucose (rapid fall after tumour removal).
- Temperature and lactate (catecholamine-driven thermogenesis).
Post-operative Care
- HDU/ICU for 24 h: invasive BP, glucose 4-hourly, serum electrolytes.
- Continue vasopressor support until vascular tone recovers.
- Resume oral α-blocker day 1 if persistent hypertension; wean over 5–7 days.
- Check plasma metanephrines at 4 weeks; lifelong annual surveillance thereafter.
Special Situations
- Pregnancy: If diagnosed ≤ 24 weeks → laparoscopic adrenalectomy after α-blockade; > 24 weeks or during labour → elective caesarean section then tumour excision. Phenoxybenzamine and metoprolol considered safe.
- Unexpected intra-operative PCC: Halt surgery, secure airway, deepen anaesthesia, start SNP / phentolamine and aggressive fluids; proceed only in experienced centre.
- Bilateral adrenalectomy: Give hydrocortisone 100 mg IV at vein ligation, then 50 mg 6-hourly; commence lifelong steroid and mineralocorticoid replacement.
Carcinoid Syndrome
[Carcinoid syndrome video
Summary
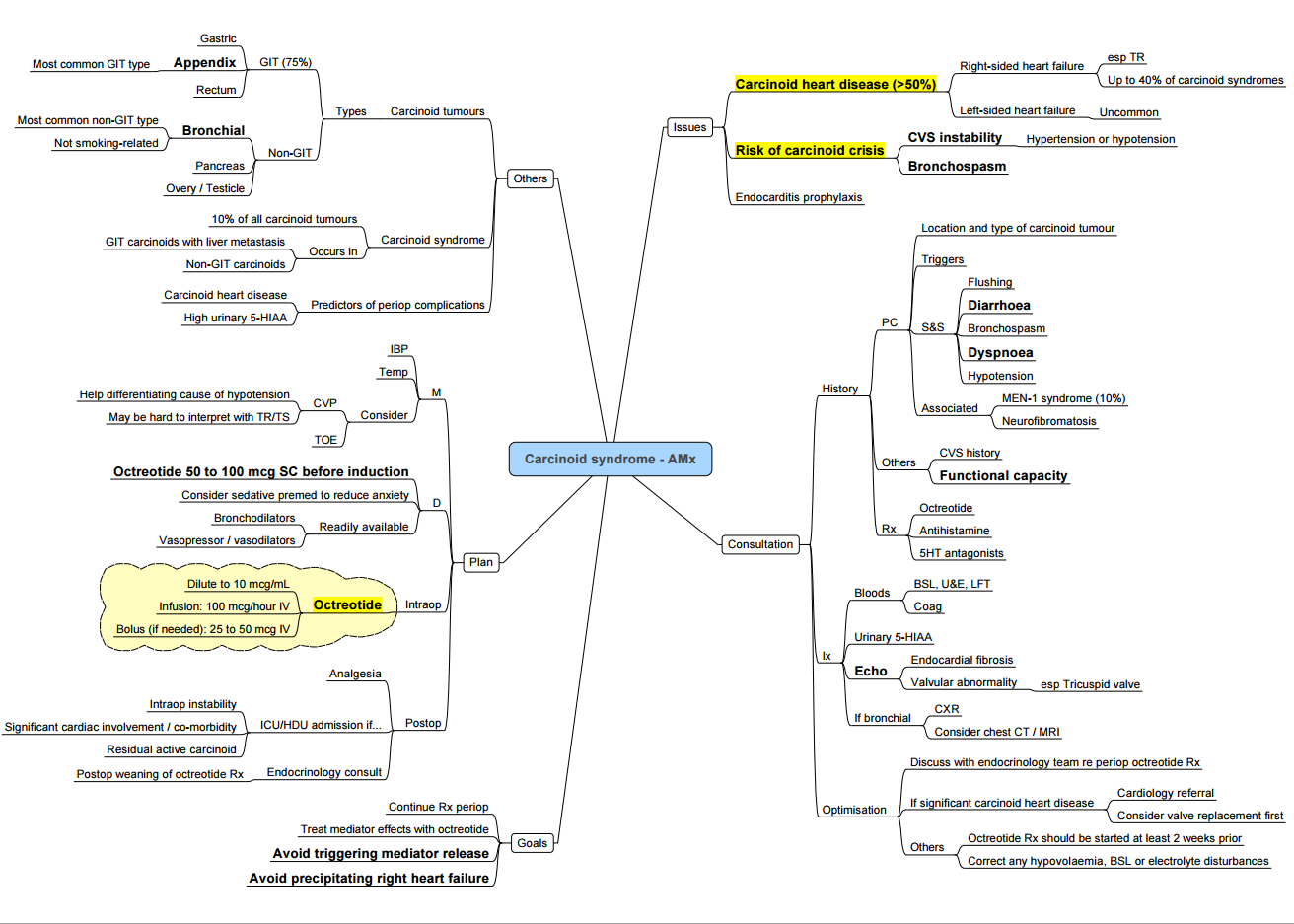
Key Points
- Vaso-active amines (mainly serotonin) released by well-differentiated neuro-endocrine tumours (NETs) cause the triad flushing ± diarrhoea ± bronchospasm; episodic hypotension or hypertension constitutes a carcinoid crisis.
- Crisis can be precipitated by anaesthesia, tumour manipulation, hypoxia, hypotension, hypercarbia, hypothermia or histamine-releasing drugs.
- Octreotide is first-line prophylaxis and treatment: start a short-acting infusion 50–100 µg h⁻¹ at least 12 h pre-operatively, give a further 100 µg IV bolus before induction, and continue through surgery; repeat 100–200 µg boluses for breakthrough symptoms
- Right-sided valvular fibrosis develops in > 50 % of patients with chronic hormone exposure; obtain an echocardiogram in all symptomatic or metastatic cases.
- Phenylephrine or vasopressin restore pressure during post-resection vasoplegia; avoid epinephrine, norepinephrine and ephedrine (may provoke paradoxical release).
Pathophysiology
| Mediator | Source | Principal effects |
|---|---|---|
| Serotonin (5-HT) | Enterochromaffin cells | Diarrhoea, abdominal cramping, coronary/mesenteric vasospasm, valvular fibrosis |
| Kallikrein → bradykinin | Tumour & peripheral conversion | Flushing, bronchospasm, hypotension |
| Histamine | Fore-gut tumours | Flushing, tachy-arrhythmia, bronchospasm |
Clinical Spectrum
- Cutaneous: episodic pink-red flushing of face, neck and upper chest; fore-gut tumours may cause prolonged violaceous flush.
- Gastro-intestinal: watery diarrhoea, abdominal pain, pellagra (niacin depletion).
- Cardiac: tricuspid and pulmonary regurgitation, right-sided heart failure, supraventricular tachycardia.
- Respiratory: wheeze due to bronchoconstriction
Diagnosis
- Biochemistry–plasma free metanephrines/chromogranin A, or 24 h urinary 5-HIAA > 50 µmol.
- Imaging–CT/MRI for localisation; ⁶⁸Ga-DOTATATE PET for occult disease.
- Cardiac–transthoracic echo for all with chronic syndrome or raised NT-proBNP (screen for carcinoid heart disease).
Peri-operative Management
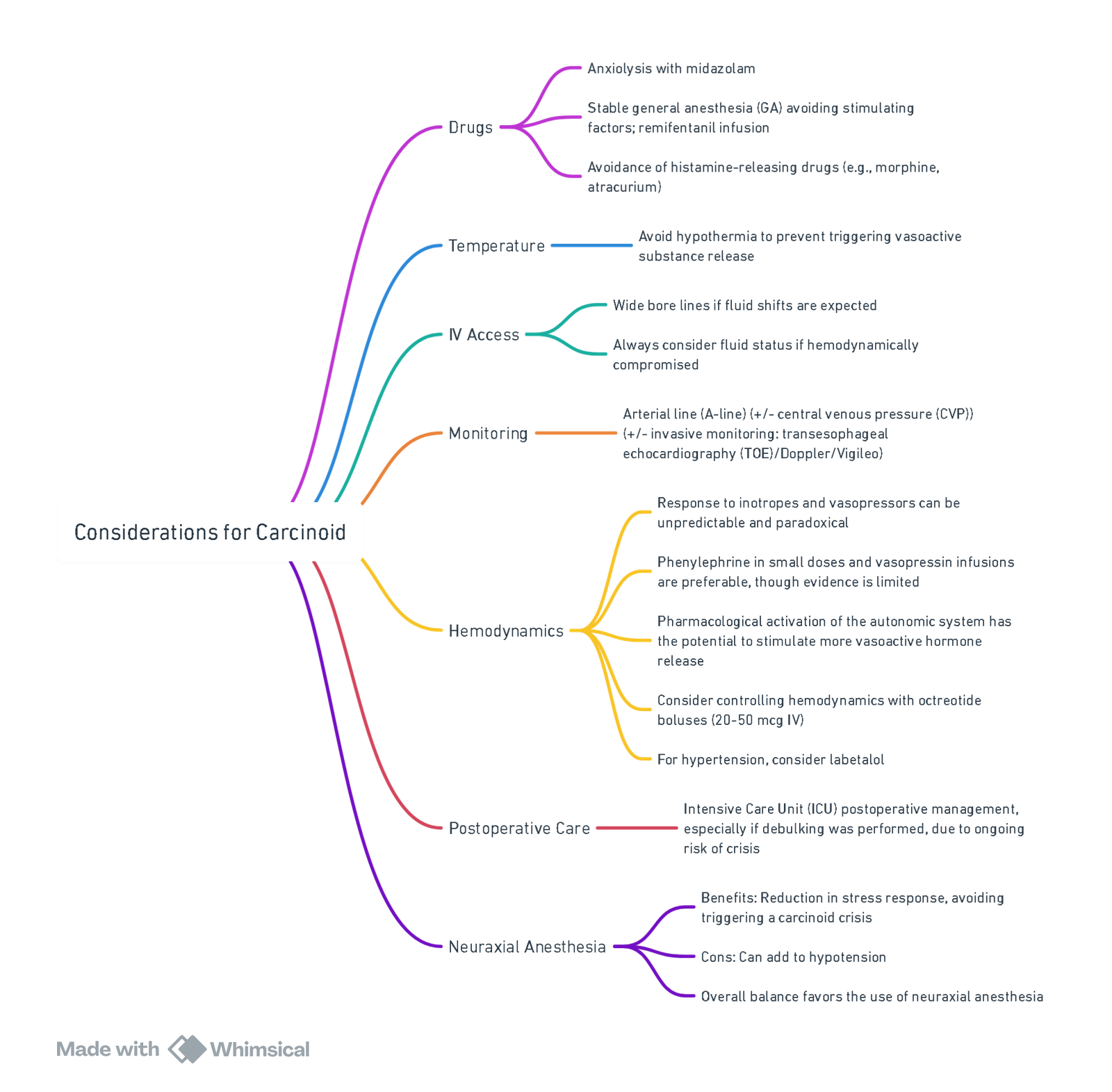
View or edit this diagram in Whimsical.
Pre-operative
| Task | Rationale |
|---|---|
| Continue long-acting somatostatin analogue (SSA) | Avoid hormone rebound |
| Start octreotide 50–100 µg h⁻¹ IV ≥ 12 h before GA | Reduces crisis incidence |
| Bolus 100 µg octreotide IV immediately pre-induction | Blunts intubation trigger |
| Correct electrolytes, niacin and iron deficiency | Optimise fluid status & nutrition |
| Echocardiography & ECG | Assess RV function, arrhythmia |
| Avoid drugs that provoke histamine (morphine, atracurium) or sympathetic surge (ketamine, desflurane induction) |
Intra-operative
- Monitoring: A-line, ± CVP; TOE if severe RH dysfunction.
- Induction: Propofol / etomidate + remifentanil; rocuronium.
- Maintenance: Sevoflurane or TIVA; BIS 40–60; keep normothermia & normocapnia.
- Crisis treatment
- Bolus octreotide 100 µg IV (repeat q 2 min).
- Persistent hypotension → phenylephrine 50–100 µg IV or vasopressin 0.5–1 U; give fluid bolus.
- Bronchospasm → octreotide bolus + H₁/H₂ blockade (diphenhydramine 25 mg, ranitidine 50 mg) + inhaled ipratropium; avoid β-agonists.
Octreotide
- Octreotide reduces vasoactive peptide release in carcinoid syndrome because it is a somatostatin analogue—a synthetic version of the naturally occurring hormone somatostatin, which broadly inhibits hormone secretion.
Mechanism of Action
- Somatostatin Receptors: Most neuroendocrine tumors, including carcinoid tumors, express somatostatin receptors, especially subtype 2 (SSTR2).
- Octreotide binds to SSTR2, mimicking somatostatin’s inhibitory effects.
- This binding inhibits the release of multiple vasoactive substances, including:
- Serotonin
- Bradykinin
- Histamine
- Prostaglandins
- Tachykinins
Result
- Reduction in hormone secretion → Improvement of symptoms
- Stabilization of tumor activity in some case
Post-operative
- HDU/ICU for at least 24 h; continue octreotide 50 µg h⁻¹ for 12–24 h then wean.
- Monitor glucose (octreotide inhibits insulin), electrolytes and lactate.
- Resume long-acting SSA on schedule; arrange NET MDT review for oncological plan.
Carcinoid Crisis: Summary Algorithm
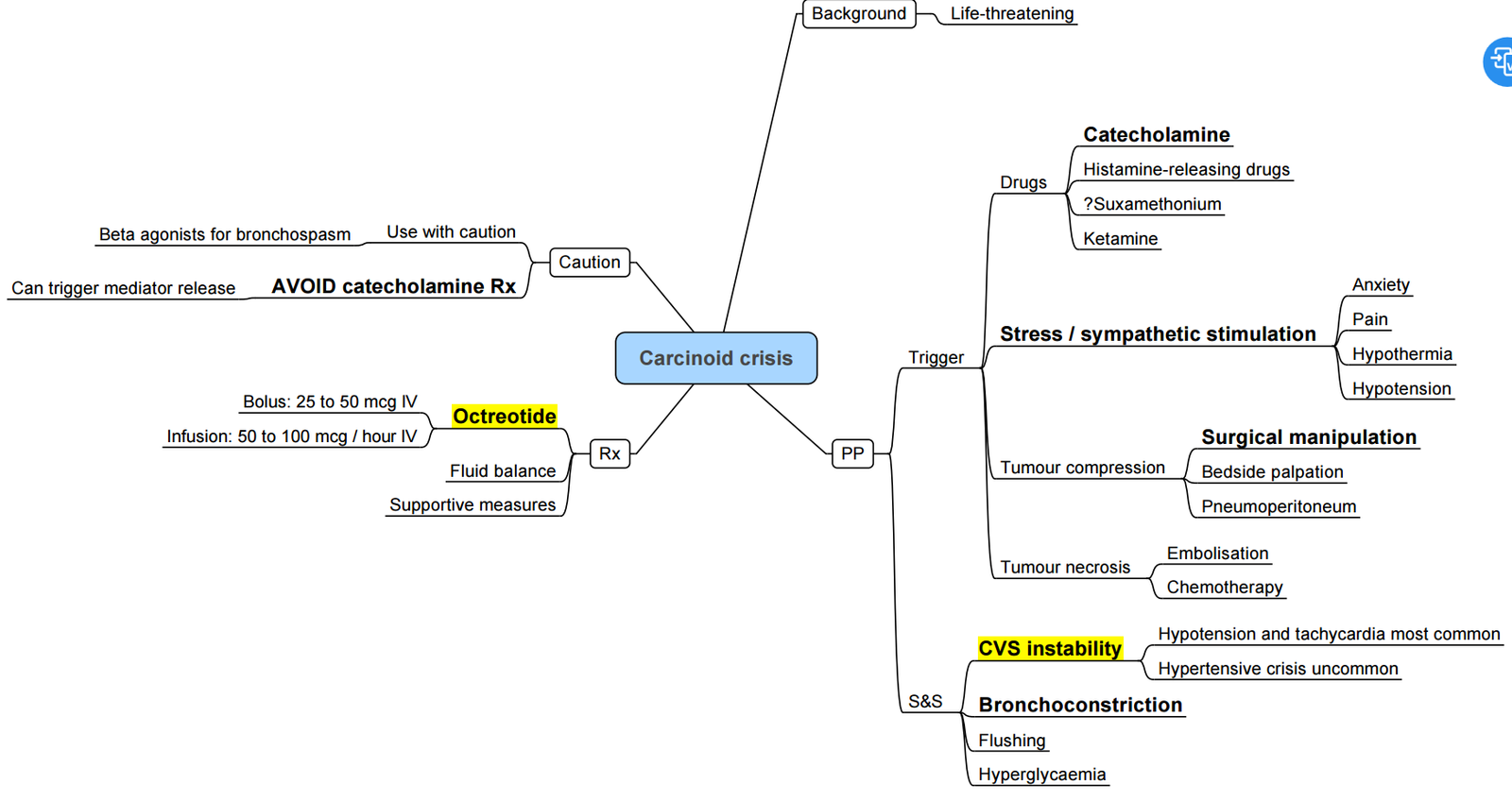
- Recognise–abrupt flushing ± hypotension/ hypertension, tachycardia, bronchospasm.
- Stop surgical stimulus where possible.
- Octreotide–100 µg IV bolus → repeat; commence/ increase infusion 50–200 µg h⁻¹.
- Supportive measures–100 % O₂, balanced crystalloid bolus, phenylephrine or vasopressin.
- Bronchospasm Adjuncts–H₁/H₂ antagonists; hydrocortisone 100 mg IV (reduce kallikrein/ bradykinin).
Avoid in Carcinoid NET Surgery
- Succinylcholine (histamine release)
- Ephedrine, epinephrine, norepinephrine, B agonists (salbutamol)
- Morphine, meperidine, atracurium
- Ketamine bolus, desflurane during rapid changes
- Hypotension, hypoxia, hypercarbia, hypothermia–all increase mediator release
Multiple Endocrine Neoplasia (MEN)
| Syndrome | Gene (inheritance) | Core tumours | Anaesthetic red flags |
|---|---|---|---|
| MEN 1 (“Wermer”) | MEN1 (AD) | Parathyroid → hyperCa²⁺, pancreatic NETs (gastrinoma, insulinoma), pituitary | Treat hypercalcaemia; screen for hypoglycaemic spells & raised ICP from pituitary macro-adenoma |
| MEN 2A | RET (AD) | Medullary thyroid carcinoma (MTC), phaeochromocytoma, parathyroid | Exclude phaeochromocytoma before any surgery; evaluate vocal cords after thyroid surgery |
| MEN 2B | RET (AD) | MTC, phaeochromocytoma, mucosal neuromas, marfanoid habitus | Anticipate difficult airway (tongue/lip neuromas), autonomic instability |
| MEN 4 | CDKN1B (rare) | Similar to MEN 1 but no MEN1 mutation | Same as MEN 1 |
Always document calcium, metanephrines and pituitary hormones in MEN patients scheduled for anaesthesia.
Acromegaly
Summary of Acromegaly
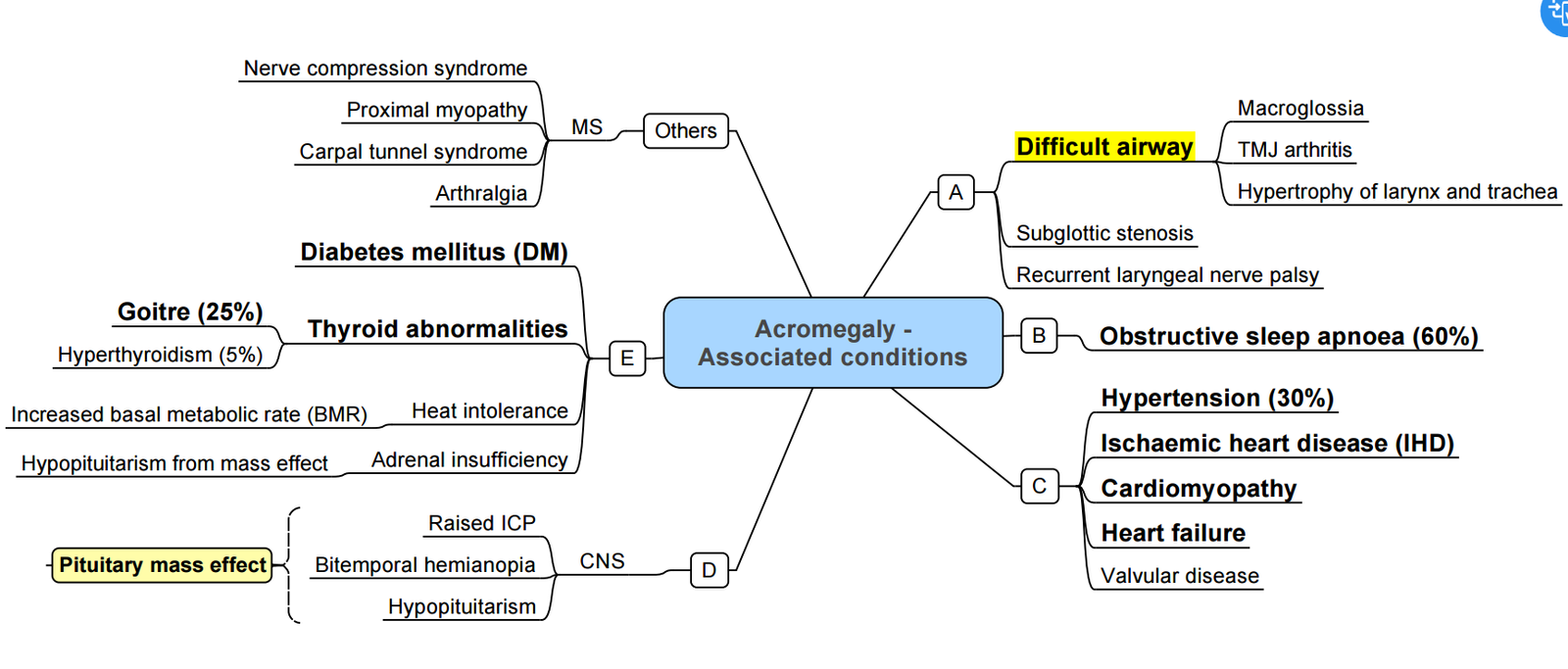
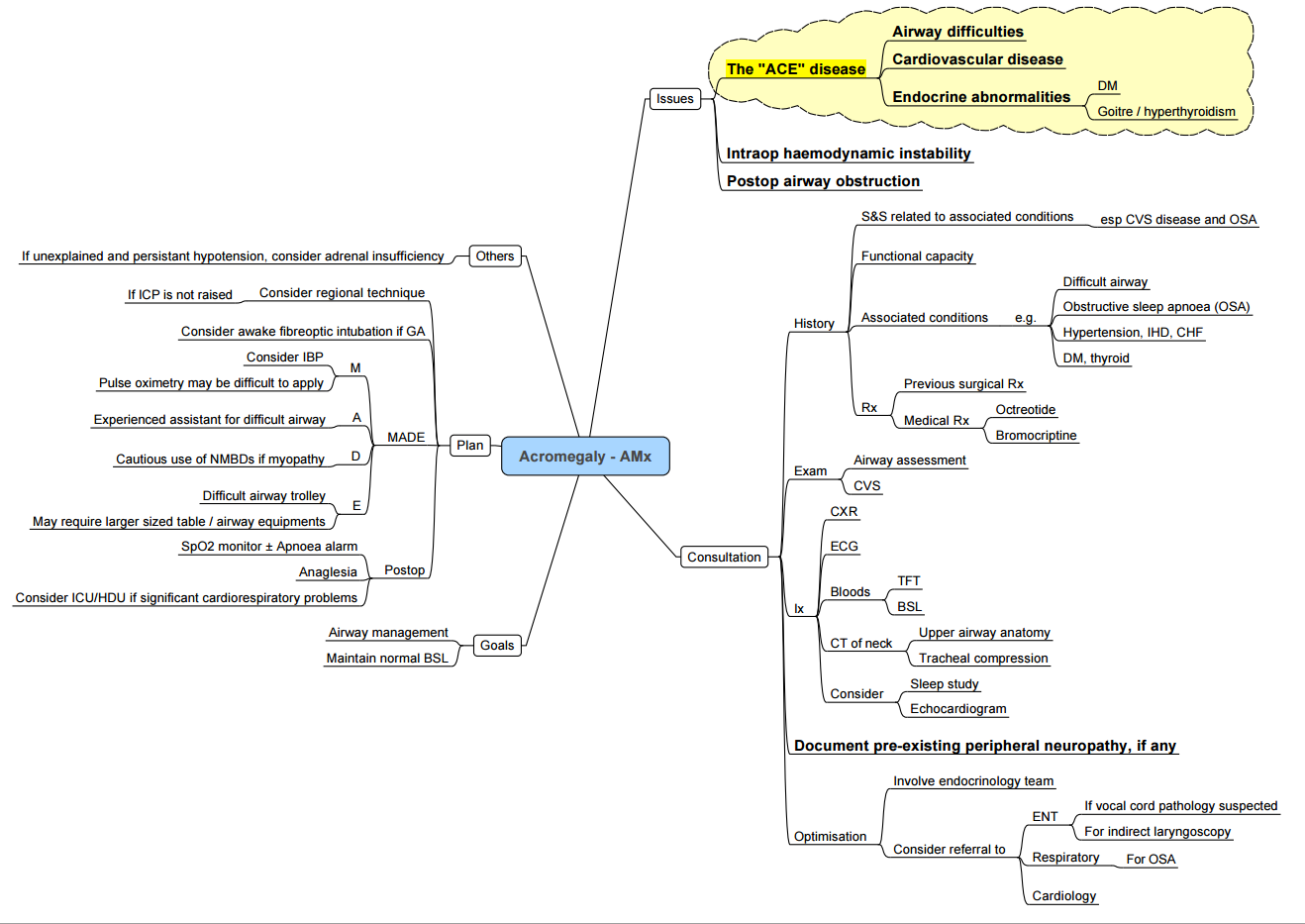
Key Peri-operative Issues
| System | Manifestations | Anaesthetic impact |
|---|---|---|
| Airway | Macroglossia, prognathism, sub-/supraglottic narrowing, large goitre (25 %) | Anticipate difficult mask & intubation; ramp, VL, smaller ETT; consider awake fibre-optic if stridor/voice change |
| CVS | HTN, LV hypertrophy, arrhythmias, cardiomyopathy | Pre-op echo if symptoms; invasive BP for trans-sphenoidal surgery |
| Respiratory | OSA (60 %), restrictive chest wall, PHT | Post-op CPAP/NIV, opioid-sparing |
| Metabolic | Insulin resistance/DM | Hourly glucose |
| MSK/soft tissue | Arthropathy, carpal tunnel, thickened skin | Positioning, arterial line collateral flow may be poor |
- Technique tips
- Avoid deep neck flexion/extension (cervical arthropathy).
- Secure IV lines; oily skin may loosen ECG electrodes.
- Neuromuscular monitoring—up to 30 % have myopathy with delayed recovery.
Anorexia Nervosa
| Problem | Anaesthetic consequence | Management |
|---|---|---|
| Severe malnutrition, BMI < 17 kg m⁻² | Hypotension, hypothermia, impaired wound healing | Warm environment, gentle positioning, early antibiotics |
| Electrolyte depletion (↓K⁺, ↓Mg²⁺, ↓PO₄³⁻) | Arrhythmia, muscle weakness, re-feeding syndrome | Correct before elective surgery; replace PO₄³⁻ & thiamine with feeds |
| Cardiac atrophy, prolonged QT | Brady-/tachy-arrhythmia, arrest on induction | Baseline ECG ± echo; avoid QT-prolonging drugs |
| Delayed gastric emptying | Aspiration | RSI with head-up; pro-kinetic |
| Psychiatric co-morbidity | Poor cooperation, medication interactions | Pre-op psych review; clear consent |
Elective surgery should be deferred if weight < 13 kg m⁻² or severe biochemistry derangements.
Diabetes Insipidus (DI)
| Type | Aetiology | Key features | Peri-op strategy |
|---|---|---|---|
| Central | ↓ ADH (pituitary / hypothalamus) | Polyuria, Uosm < 150 mOsm kg⁻¹ | Desmopressin 1 µg IV q8-12 h; monitor Na⁺ 2-hourly |
| Nephrogenic | Renal resistance to ADH | Same biochemistry + high ADH | Continue thiazide ± amiloride; match urine with 0.45 % NaCl |
- Treat hypernatraemia slowly (< 10 mmol L⁻¹ day⁻¹) to avoid cerebral oedema.
- Anticipate ↑ MAC requirement if Na⁺ > 155 mmol L⁻¹.
SIADH
Key Anaesthetic Concerns
- Chronic hyponatraemia (< 130 mmol L⁻¹) increases risk of postoperative delirium, seizures and delayed emergence.
- Over-rapid correction (> 8 mmol L⁻¹ in any 24 h) risks osmotic demyelination syndrome (ODS).
Diagnostic Criteria (euvolaemic hyponatraemia)
- Serum osmolality < 275 mOsm kg⁻¹.
- Urine osmolality > 100 mOsm kg⁻¹ with urine Na⁺ > 40 mmol L⁻¹.
- Normal thyroid, adrenal and renal function.
Peri-operative Management
| Situation | Treatment |
|---|---|
| Elective surgery, Na⁺ < 125 mmol L⁻¹ | Defer; fluid restrict (800–1000 mL day⁻¹) ± loop diuretic; treat cause. |
| Moderate symptoms (confusion, nausea) | 3 % saline bolus 150 mL over 15 min; aim ΔNa⁺ + 4–6 mmol L⁻¹. Re-bolus if symptoms persist. |
| Severe symptoms (seizure, coma) | Two 150 mL 3 % saline boluses; monitor Na⁺ hourly. Add furosemide 20 mg IV if volume overloaded. |
| Chronic phase | Restrict water, consider oral urea, demeclocycline or vasopressin V₂ antagonist (tolvaptan) under specialist advice. |
- Correction targets–maximum 10 mmol L⁻¹ first 24 h and 8 mmol L⁻¹ each subsequent 24 h; high-risk groups (malnutrition, alcohol use, hypokalaemia, liver disease) max 8 mmol L⁻¹ day⁻¹ from outset.
In Theatre
- Check plasma Na⁺ on arrival and 2-hourly intra-operatively.
- Avoid hypotonic fluids; use isotonic balanced crystalloids.
- Treat convulsions with benzodiazepine; give small hypertonic saline bolus rather than large continuous infusion.
Panhypopituitarism
- Hormone cover essentials
- Glucocorticoid–hydrocortisone 25 mg PO morning dose; give stress dose for surgery (see table below).
- Thyroxine–continue usual dose; never start before hydrocortisone in new diagnoses.
- Consider vasopressin deficiency (monitor urine output & Na⁺), sex-steroid replacement and GH in long-term care. Sudden hypotension or hypoglycaemia warrants immediate hydrocortisone 100 mg IV.
Porphyria
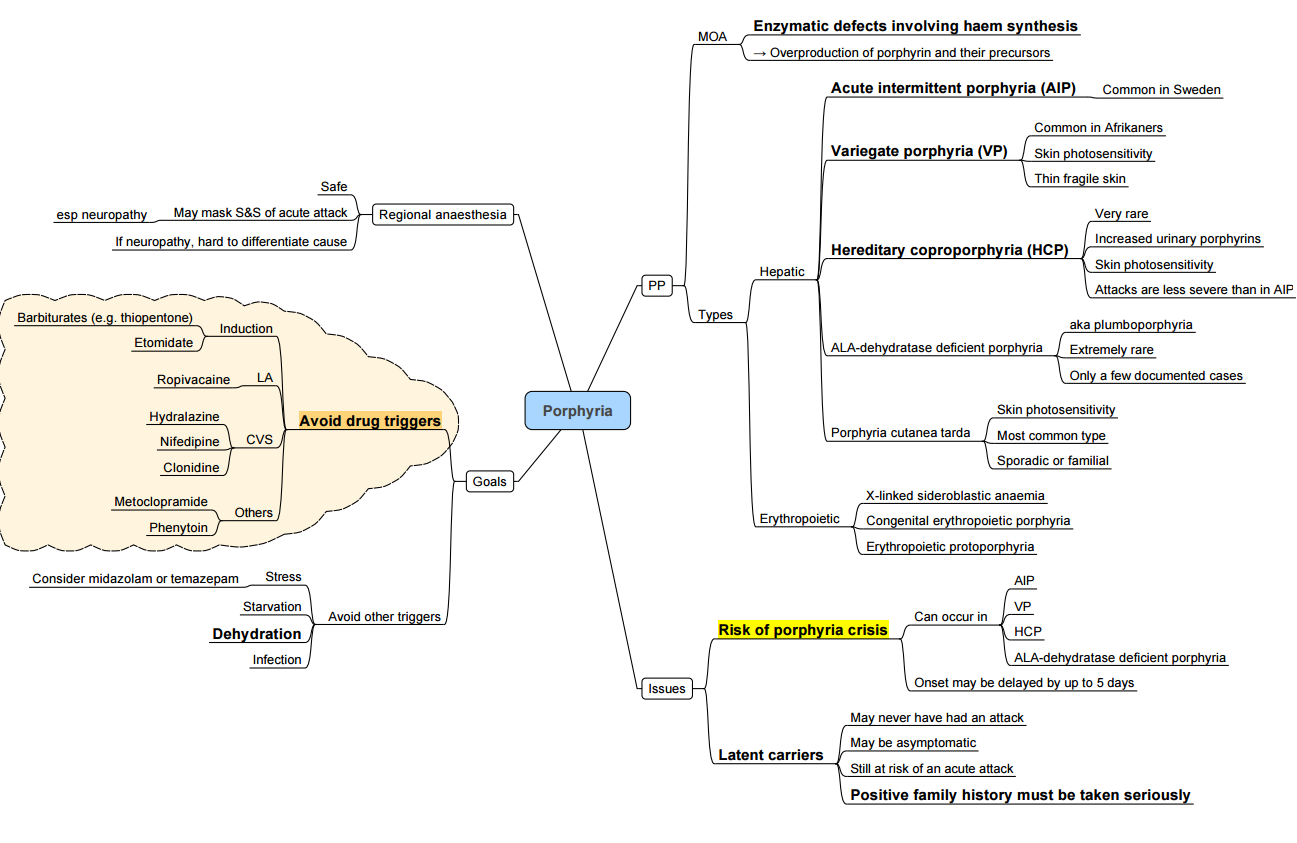
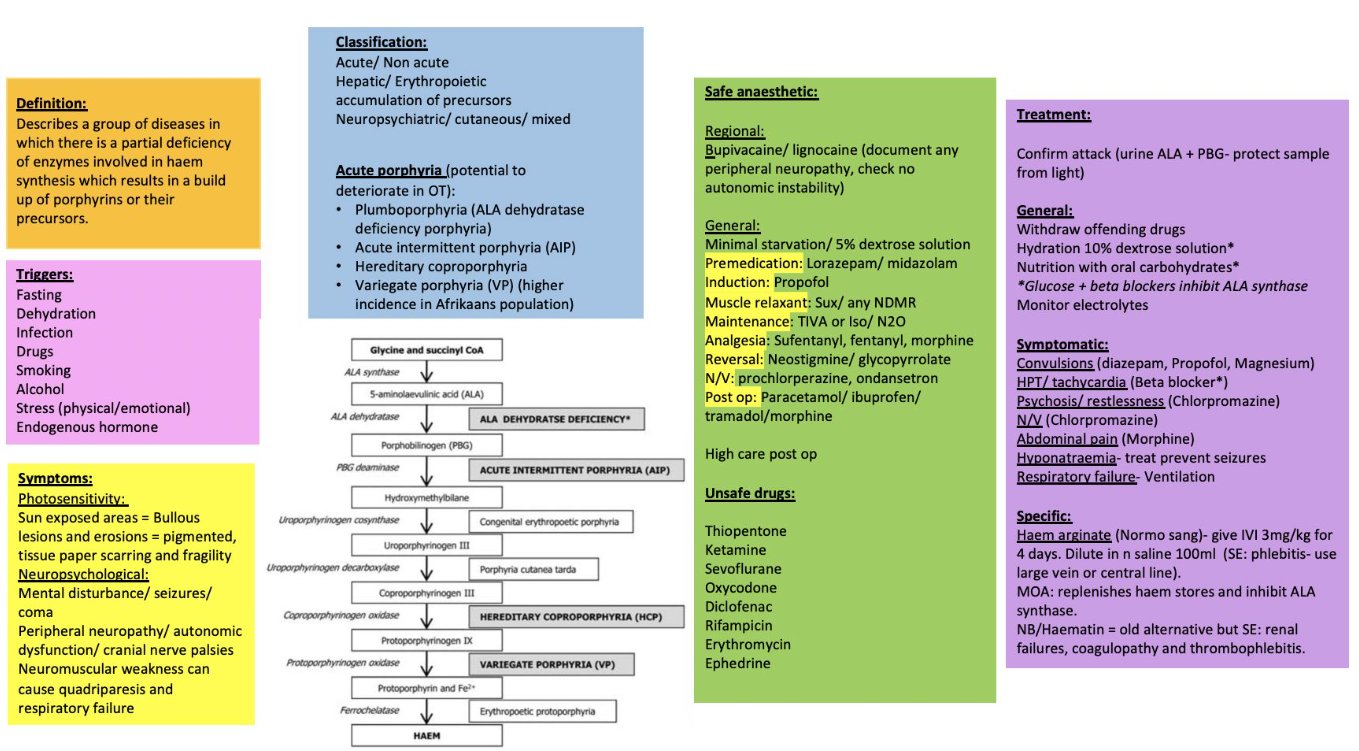
Key Points
- Acute hepatic porphyrias (AIP, variegate, hereditary coproporphyria, ALAD‐deficiency) may be triggered by porphyrinogenic drugs, fasting, infection, alcohol and stress
- A safe anaesthetic hinges on (1) avoiding unsafe drugs, (2) preventing physiological triggers and (3) being able to recognise and treat an acute attack swiftly.
Clinical Spectrum
| Phase | Features |
|---|---|
| Acute attack | Severe abdominal pain, vomiting, autonomic instability (tachycardia ± hypertension), hyponatraemia/SIADH, motor neuropathy → respiratory failure, seizures |
| Chronic sequelae | Recurrent pain, neuropathic pain, chronic kidney disease, hypertension, anxiety & depression |
Peri-operative Management
Pre-operative
- Haematology consultation; ensure intravenous haem arginate (hemin) 3 mg kg⁻¹ is immediately available.
- Continue high-carbohydrate diet; if NPO infuse 10 % dextrose-saline 1.5 mL kg⁻¹ h⁻¹.
- Correct hyponatraemia, anaemia and treat infection.
- Avoid prolonged fasting, dehydration and oestrogen-containing drugs.
Drug Safety (2024 Welsh National Acute Porphyria Service list)
| Category | SAFE / PREFERRED | UNSAFE–AVOID |
|---|---|---|
| Induction | Propofol, Ketamine (single dose), Midazolam | Thiopentone, Etomidate† |
| Inhalational | Sevoflurane, Desflurane, Isoflurane, N₂O | Enflurane, Methoxyflurane |
| Analgesia | Fentanyl, Remifentanil, Morphine, Paracetamol, Ketamine | Diclofenac, Ketorolac |
| Muscle relaxants | Suxamethonium, Rocuronium, Cis-/Atracurium | Pancuronium (limited data) |
| Local anaesthetics | Lidocaine, Bupivacaine, Levobupivacaine, Ropivacaine‡ | Prilocaine (limited data) |
| Antiemetic | Ondansetron, Dexamethasone | Metoclopramide* |
| Vasopressors | Phenylephrine, Vasopressin | Ephedrine |
| Others | Antibiotics: all β-lactams, clindamycin, ciprofloxacin safe | Rifampicin, Sulphonamides |
† Etomidate probably safe in single dose but lacks robust data—prefer alternatives.
‡ Safe in large contemporary series; classify as “probably safe”.
Intra-operative Checklist
- Standard monitors + arterial line if autonomic instability anticipated.
- Maintain normoglycaemia and normothermia.
- Minimise surgical stress: adequate depth (BIS 40–60), analgesia and anti-emesis.
- Avoid hyperventilation-induced alkalosis (may precipitate attack).
Treating an Acute Porphyric Crisis
- Stop precipitating drug / stimulus.
- Haem arginate (hemin) 3 mg kg⁻¹ IV over 20 min daily × 4 days.
- Glucose 10 % infusion 200 g day⁻¹.
- Symptom control: opiate analgesia, midazolam / propofol for seizures (avoid phenytoin)
- Correct hyponatraemia slowly; treat hypertension/tachycardia with short-acting β-blocker
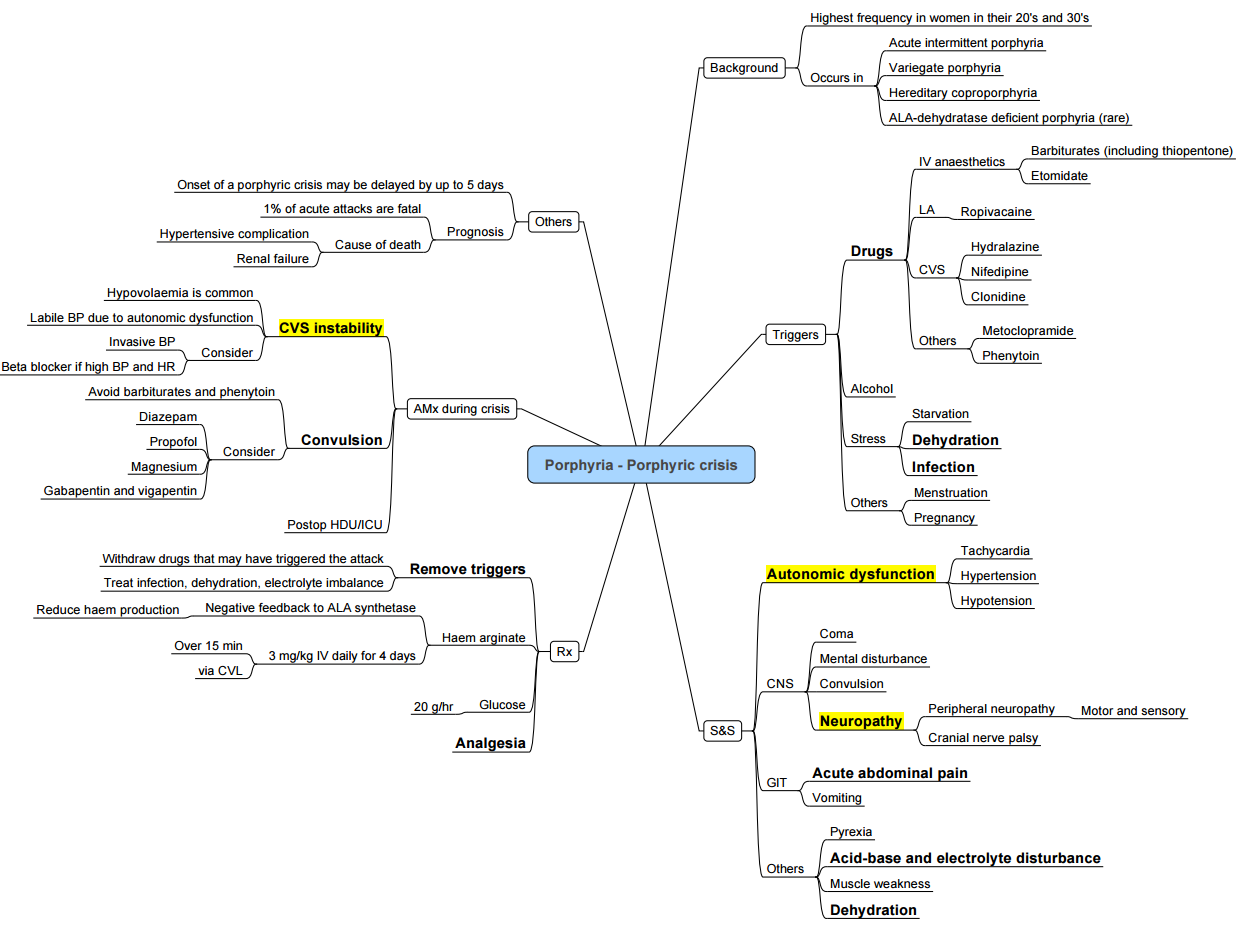
Summary of a Safe Anesthetic
View or edit this diagram in Whimsical.
Summary of Drugs
- Avoid: Thiopentone, Sevoflurane, Ketamine, Diclofenac, Oxycodone, Rifampicin, Erythromycin, Ephedrine (TORE DESK).
- Caution: Etomidate, Levobupivicaine, Ropivacaine, Pentazocine, Mefenamic Acid, Vasopressin, Metaraminol, Dexamethasone, Hydrocortisone, Clopidogrel (HELD M CRAMP).
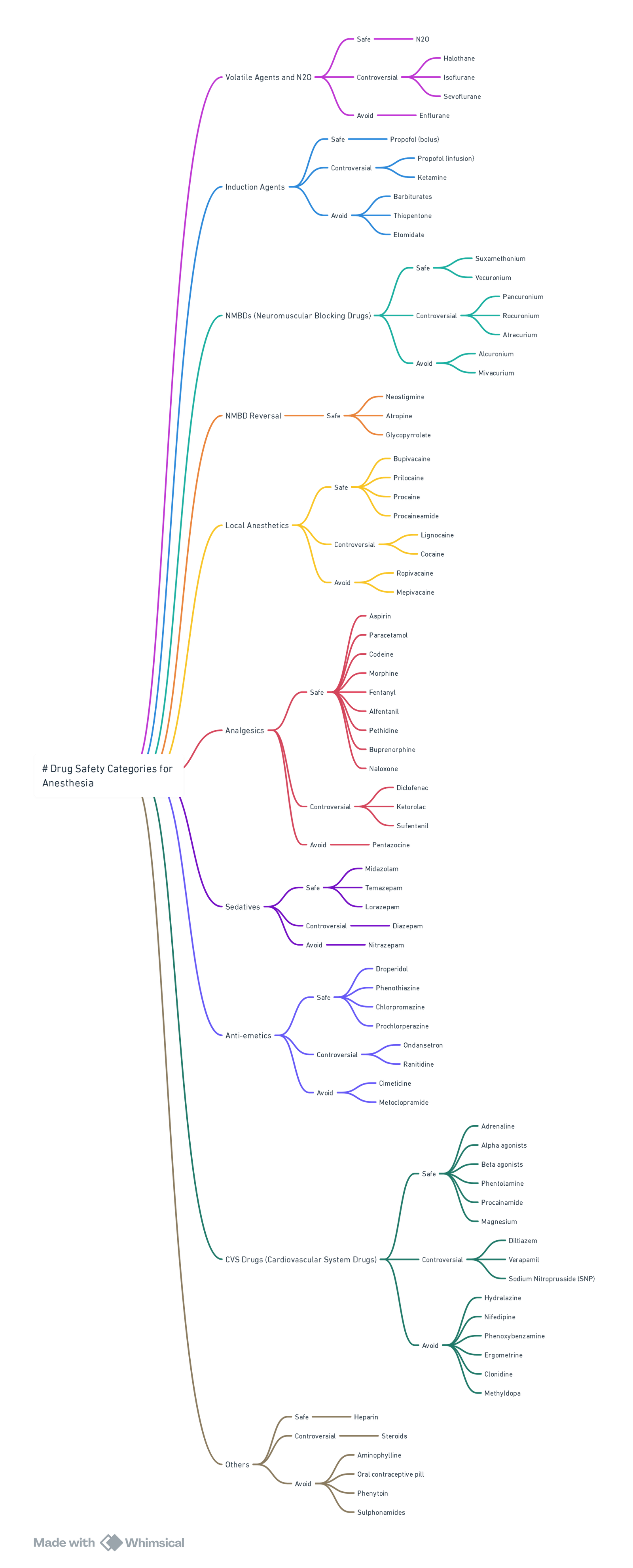
View or edit this diagram in Whimsical.
Links
Past Exam Questions
Airway Obstruction After Thyroidectomy
Briefly discuss the potential causes of airway obstruction after a thyroidectomy and how they typically present. (10)
Anaesthesia for Acute Porphyria
a) The acute types of porphyria can complicate into an acute neurovisceral crisis.
- i) List 4 triggers for acute neurovisceral crisis in the perioperative period. (2)
- ii) List 2 cardiovascular symptoms that manifest during an acute crisis. (1)
b) List the 3 mechanisms of drug porphyrinogenicity. (3)
c) How will you manage an acute porphyria crisis? (4)
References:
- Crowley K, Scanaill PÓ, Hermanides J, Buggy DJ. Current practice in the perioperative management of patients with diabetes mellitus: a narrative review. British Journal of Anaesthesia. 2023 Aug;131(2):242-252. DOI: 10.1016/j.bja.2023.02.039. PMID: 37061429.
- Chan S Y et al. High-flow nasal oxygen for shared airway procedures: systematic review and meta-analysis. Anaesth Intensive Care. 2024;52:123-33. pmc.ncbi.nlm.nih.gov
- Fugazzola L et al. 2022 European Thyroid Association guideline for management of Graves’ hyperthyroidism. Eur Thyroid J. 2022;11:e210046. pmc.ncbi.nlm.nih.gov
- Wong R, See T. Airway management in retrosternal goitre: review of 22 cases. Tech Respir Care Med. 2022;26:55-63. pmc.ncbi.nlm.nih.gov
- Johnson P J P et al. Awake versus asleep intubation for mediastinal goitres: systematic review. Anesth Analg. 2025;140:889-900. pmc.ncbi.nlm.nih.gov
- Meng L et al. High-flow nasal oxygen during suspension laryngoscopy: randomised controlled trial. J Int Med Res. 2023;51:300605052. journals.sagepub.com
- Schneider R et al. Intra-operative neuromonitoring and recurrent laryngeal nerve injury: meta-analysis of 60 studies. Diagnostics. 2023;13:860. mdpi.com
- Lin Y C et al. Endoscopic evidence of RLN outcomes with and without IONM: systematic review. Front Endocrinol. 2023;14:11507766. pmc.ncbi.nlm.nih.gov
- Kim H G et al. Effects of different cisatracurium doses on RLN monitoring during thyroid surgery: RCT. Br J Anaesth. 2021;126:120-8. bjanaesthesia.org
- van der Pas N et al. Risk of peri-operative thyroid storm in hyperthyroid patients: systematic review. Br J Anaesth. 2021;127:423-33. bjanaesthesia.org
- Oettlé M et al. Anaesthesia for retrosternal thyroidectomy: South African cohort review. S Afr J Anaesth Analg. 2023;29:256-62. sajaa.co.za
- StatPearls Publishing. Thyroid storm. Updated 2024 Jan. ncbi.nlm.nih.gov
- Malhotra B, Bhadada SK. Perioperative management for non-thyroidal surgery in thyroid dysfunction. Indian J Endocrinol Metab. 2022;26:428-434. pmc.ncbi.nlm.nih.gov
- Elshimy G, Chippa V, Correa R. Myxedema. StatPearls. 2023 Aug 14. ncbi.nlm.nih.gov
- Ha J et al. 2023 Korean Endocrine Society consensus guidelines for the diagnosis and management of primary aldosteronism. Endocrinol Metab (Seoul). 2023;38:597-618. pubmed.ncbi.nlm.nih.gov
- Society for Endocrinology. Adrenal Crisis–Clinical Guidance. Updated 2024. endocrinology.org
- Lenders J WM, Duh Q-Y, Eisenhofer G, et al. Phaeochromocytoma and paraganglioma: Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-42. pubmed.ncbi.nlm.nih.gov
- StatPearls Publishing. Pheochromocytoma. Updated 2024 Oct. ncbi.nlm.nih.gov
- StatPearls Publishing. Peri-operative management of pheochromocytoma. Updated 2023 Sep. statpearls.com
- Casey RT, Hendriks E, Deal C, et al. International consensus on phaeochromocytoma & paraganglioma in children and adolescents. Nat Rev Endocrinol. 2024;20:729-48. nature.com
- Gombert AJ et al. Peri-operative biochemical and clinical considerations in pheochromocytoma. Int J Mol Sci. 2023;26:6080. mdpi.com
- Association of Anaesthetists & Royal College of Physicians. Guidelines for the management of glucocorticoids during the peri-operative period for patients with adrenal insufficiency. Anaesthesia. 2020. pubmed.ncbi.nlm.nih.gov
- Villaseñor M et al. Peri-operative management of Cushing disease. J Endocr Soc. 2022;6(4):bvac185. pmc.ncbi.nlm.nih.gov
- Eledrisi MS. Myxedema coma or crisis: treatment & management. Medscape. Updated 12 Jan 2024. emedicine.medscape.com
- OpenAnesthesia. Hypothyroidism. Updated 23 May 2025. openanesthesia.org
- Pavel M, et al. European Neuroendocrine Tumour Society guidance for carcinoid syndrome and carcinoid heart disease. J Neuroendocrinol. 2022;34:e13146. onlinelibrary.wiley.com
- UKINETS & BSG. Peri-operative management of patients with NETs. 2023. ukinets.org
- StatPearls Publishing. Carcinoid Syndrome. Updated 2024 Jan. ncbi.nlm.nih.gov
- OpenAnesthesia. Carcinoid Syndrome–Anaesthetic considerations. Updated 2023 Sep. openanesthesia.org
- Hallett J, et al. Peri-operative management of carcinoid crisis: expert consensus protocol. Eur J Surg Oncol. 2023;49:2285-92. magonlinelibrary.com
- Society for Obesity & Bariatric Anaesthesia. SOBA-UK Adult Peri-operative Obesity Guideline. 2022. sobauk.co.uk
- Society for Obesity & Bariatric Anaesthesia. SOBA-UK Paediatric Obesity Guideline. 2024. sobauk.co.uk
- Ushakumari DS, Sladen RN. ASA consensus guidance on peri-operative management of GLP-1 receptor agonists. Anesthesiology. 2024;140:346-8. pubmed.ncbi.nlm.nih.gov
- Schutzer-Weissmann J, et al. Apnoeic oxygenation in morbid obesity: HFNO vs facemask. Br J Anaesth. 2023;130:103-110. pubmed.ncbi.nlm.nih.gov
- Lee J H, et al. High-flow nasal cannula for apnoeic oxygenation in obese adults: systematic review & meta-analysis. Anesth Analg. 2023;136:483-493. journals.lww.com
- Samantray A, et al. Propofol induction dosing based on lean body weight vs fat-free mass in morbid obesity: randomised trial. Anaesthesia. 2023;78:1052-1060. pmc.ncbi.nlm.nih.gov
- Lyons PG, et al. Peri-operative management of obstructive sleep apnoea: narrative review. Br J Anaesth. 2023;131:887-901. bjanaesthesia.org.uk
- Butterworth J, Mackey D, Wasnick J. Morgan and Mikhail’s Clinical Anesthesiology, 7th Edition. 7th edition. New York: McGraw Hill Medical; 2022.
- Allman K, Wilson I, O’Donnell A. Oxford Handbook of Anaesthesia. Vol. 4. Great Clarendon Street, Oxford, OX2 6DP, United Kingdom: Oxford University Press; 2016. 1295 p.
- Children’s Hospital of Philadelphia. MEN2 peri-operative screening guidance. 2023. chop.edu
- Fleseriu M, et al. Consensus on acromegaly diagnosis & management. Endocrine 2024;20:29–45. karger.com
- BJA Education. Anaesthetic implications of acromegaly. 2023;23:210–17. academic.oup.com
- McGuire E, et al. Anaesthesia for patients with anorexia nervosa. BJA Educ. 2023;23:18–25. bjaed.orgpmc.ncbi.nlm.nih.gov
- Refardt J, Christ-Crain M. Diagnosis & management of central diabetes insipidus. J Clin Endocrinol Metab. 2023;107:2701-15. academic.oup.com
- Association of Anaesthetists & RCP. Management of glucocorticoids during the peri-operative period. Anaesthesia. 2020;75:654-63. anaesthetists.org
- PREdS collaborative. High vs low-dose peri-operative steroids: RCT protocol. Anaesthesia. 2024;79:1050-8. associationofanaesthetists-publications.onlinelibrary.wiley.com
- Isberner N, Nydegger M. 2024 Safe list of drugs in acute porphyria. Welsh Medicines Advice Service; 2024. wmic.wales.nhs.uk
- Floderer T, Klohr S. Anaesthesia in acute hepatic porphyria—a practical approach. Br J Anaesth Educ. 2025;25:182-9. pmc.ncbi.nlm.nih.gov
- Stewart TG, et al. Safe use of sevoflurane in patients with acute intermittent porphyria: systematic review. Front Anaesth. 2023;2:10002. frontiersin.org
- Spasovski G, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia—2024 update. Nephrol Dial Transplant. 2024;39:1583-99. academic.oup.com
- Aijaz N, Sterns RH. Rapid correction of hyponatraemia and risk of ODS: 2024 perspective. Kidney Med. 2024;6:100953.
- The Calgary Guide to Understanding Disease. (2024). Retrieved June 5, 2024, from https://calgaryguide.ucalgary.ca/
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org/
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
- ICU One Pager. (2024). Retrieved June 5, 2024, from https://onepagericu.com/
Summaries
Endocrine
Endocrine physiology
Obesity
Bariatric surgery
Anorexia
Ketoacidosis_ICU OP
Copyright
© 2025 Francois Uys. All Rights Reserved
id: “d7ceb19a-6fb1-464c-be94-14db9cef0cf8”



