- Kidney Functions
- Nephron Structure and Types
- Renal Blood Flow (RBF)
- Renal Plasma Flow (RPF)
- Glomerular Filtration Rate (GFR)
- Control Mechanisms Regulating RBF
- Links
{}
Kidney Functions
- Fluid Regulation
- The kidneys maintain the volume and composition of body fluids by filtering plasma, excreting waste products, and regulating water, osmolality, electrolytes, and acid-base balance.
- Renin Secretion
- Renin is secreted by the kidneys and is vital for blood pressure and fluid balance regulation.
- Erythropoietin Production
- Erythropoietin, essential for red blood cell production, is secreted by the kidneys.
- Vitamin D Activation
- The proximal renal tubules convert 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol (Vitamin D), which is critical for calcium homeostasis.
- Drug Handling
- The kidneys are involved in the metabolism and excretion of various drugs.
Acid-Base Balance
- Hydrogen Ion (H⁺) Secretion: H⁺, minimally filtered by the glomerulus, is secreted actively through:
- Primary active H⁺ ATPase pumps.
- Sodium-hydrogen (Na⁺/H⁺) countertransport.
- H⁺/K⁺ ATPase mechanisms.
- Bicarbonate (HCO₃⁻) Reabsorption: Filtered bicarbonate requires reabsorption facilitated by “recycling” H⁺ to reabsorb HCO₃⁻ effectively.
Nephron Structure and Types
Types of Nephrons
- Cortical Nephrons:
- Have short loops of Henle extending into the superficial renal medulla, often lacking a thin ascending limb.
- Constitute approximately 85% of all nephrons.
- Juxtamedullary Nephrons:
- Possess renal corpuscles close to the medulla.
- Feature long loops of Henle that penetrate deep into the renal medulla, contributing to concentrated urine production.
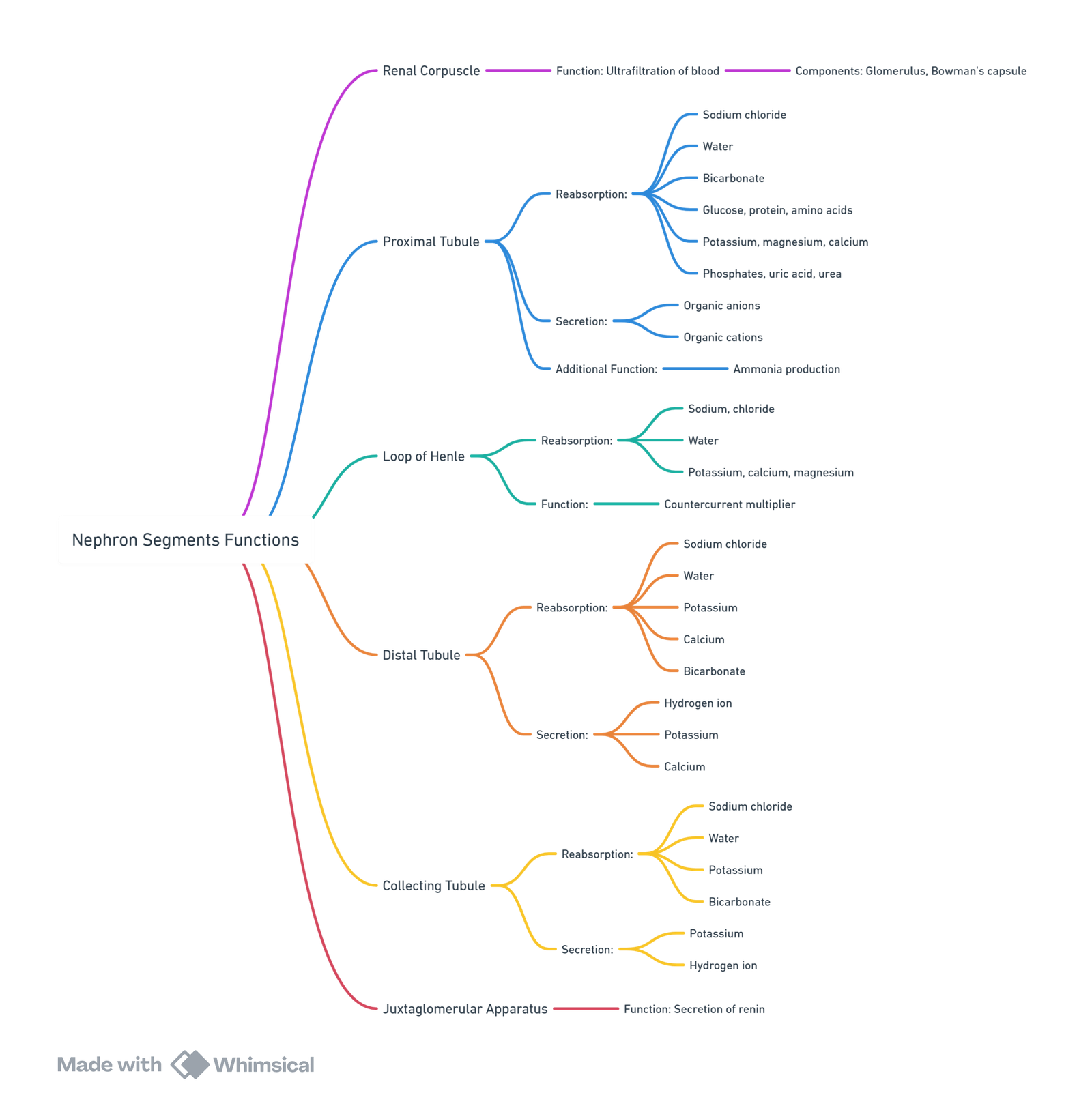
Nephron Components
- Renal Corpuscle
- Proximal Convoluted Tubule
- Loop of Henle
- Distal Renal Tubule
- Collecting Tubule
- Juxtaglomerular Apparatus
Nephron Segments
View or edit this diagram in Whimsical.
Sodium Reabsorption Across the Nephron
- Glomerulus:
- Initial filtration of sodium into the nephron.
- Proximal Convoluted Tubule:
- Reabsorbs 65–75% of filtered sodium.
- Loop of Henle (Thick Ascending Limb):
- Reabsorbs 15–20% of filtered sodium.
- Distal Convoluted Tubule:
- Reabsorbs 5–7% of filtered sodium.
- Collecting Tubule:
- Reabsorbs 5–7% of filtered sodium.
- Urinary Sodium:
- Minimal sodium remains in urine due to efficient reabsorption along the nephron.
Renal Corpuscle
Structure and Function
- Components: Includes the glomerulus and Bowman capsule.
- Location: Entirely within the renal cortex.
- Function: Produces ultrafiltrate, which flows through nephron tubules for modification into urine.
- Blood Flow:
- Blood enters through the afferent arteriole and exits via the efferent arteriole.
- Reabsorption involves movement of substances from the tubular lumen into peritubular capillaries, while secretion involves transport from blood into the tubular lumen.
Histological Features
- Cell Types:
- Glomerular Endothelial Cells: Fenestrated (70–100 nm) to allow selective filtration.
- Epithelial Cells of Bowman’s Capsule: Form filtration slits (~25 nm).
- Intraglomerular Mesangial Cells: Contractile and phagocytic cells regulating glomerular blood flow.
- Basement Membrane:
- Negatively charged, preventing passage of large molecules and cells.
Glomerular Filtration Pressure
- Hydrostatic Pressure: ~60 mmHg (60% of MAP).
- Opposition: Plasma oncotic pressure (~25 mmHg) and renal interstitial pressure (~10 mmHg).
- Net Filtration: Proportional to efferent arteriolar tone and inversely proportional to afferent tone.
Proximal Tubule
Reabsorption Functions
- Reabsorbs 65–75% of filtrate isotonically, with a focus on sodium.
Mechanisms
- Na⁺-K⁺-ATPase Pump: Creates a gradient favoring Na⁺ entry into cells, facilitating reabsorption of other ions (K⁺, Ca²⁺, Mg²⁺).
- Passive Na⁺ Movement: Enhanced by angiotensin II and norepinephrine; inhibited by dopamine and fenoldopam.
- Coupled Transport: Na⁺ reabsorption linked with phosphate, glucose, and amino acids.
- H⁺ Countertransport: Enables 90% reabsorption of bicarbonate.
Other Molecules
- Chloride: Passively reabsorbed.
- Water: Moves through aquaporin-1 channels.
- Organic Cations and Anions: Share secretory pathways, leading to competitive inhibition.
- Low-Molecular-Weight Proteins: Reabsorbed for intracellular metabolism.
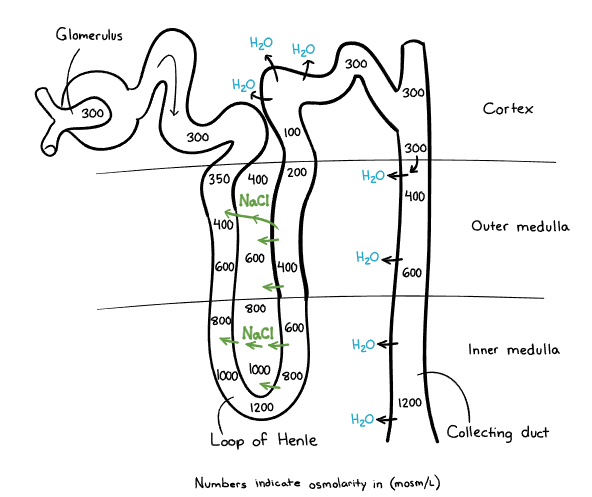
Loop of Henle
Anatomy and Functions
- Structure: Divided into descending and ascending limbs.
- Function: Maintains hypertonic medullary interstitium and concentrates urine
Reabsorption
- Filtrate Processed: ~25–35% of ultrafiltrate reaches the loop.
- The descending limb is permeable to water but impermeable to solutes (such as Na⁺ and Cl⁻). As the filtrate travels down, water is passively reabsorbed into the hypertonic medullary interstitium, concentrating the tubular fluid. This results in progressively increasing osmolality of the filtrate as it moves deeper into the medulla.
- Thick Ascending Limb: Reabsorbs 15–20% of filtered Na⁺ and Cl⁻; impermeable to water, leaving hypotonic fluid exiting the loop.
Countercurrent Mechanism
- Key Characteristics: Differential permeability between descending and ascending limbs.
- This mechanism enables the kidney to produce concentrated urine when needed
- Countercurrent Multiplication
- The descending limb passively loses water to the hypertonic medullary interstitium, concentrating the filtrate.
- The thick ascending limb actively reabsorbs Na⁺ and Cl⁻ but is impermeable to water, diluting the filtrate.
- This creates a progressively increasing osmotic gradient in the medulla.
- Countercurrent Exchange (Vasa Recta):
- The vasa recta preserves the medullary gradient by allowing passive water and solute exchange, preventing washout.
Distal Convoluted Tubule
Functions
- Processes hypotonic fluid from the loop of Henle.
- Reabsorbs ~5% of Na⁺ via Na⁺-Cl⁻ symporters, influenced by Na⁺ delivery.
Calcium Reabsorption
- Site of Action: Major site for parathyroid hormone (PTH) and vitamin D-mediated calcium reabsorption.
Collecting Tubule
Role in Osmoregulation
- Regulates medullary hypertonicity via variable urea permeability.
Types and Functions
- Cortical Collecting Tubule:
- Principal Cells: Aldosterone-driven Na⁺ reabsorption and K⁺ secretion.
- Intercalated Cells: Maintain acid-base balance by bicarbonate ion secretion.
- Medullary Collecting Tubule:
- ADH Action: Aquaporin-2 channels reabsorb water, concentrating urine during dehydration.
Juxtaglomerular Apparatus and RAAS
Components
- Specialized structure containing juxtaglomerular cells and the macula densa.
RAAS Activation
- Stimuli: Decreased blood pressure, low renal perfusion, and sympathetic activation.
- Renin Secretion: Released by juxtaglomerular cells, converting angiotensinogen (from the liver) to angiotensin I.
- ACE Function: Converts angiotensin I to angiotensin II in the lungs and kidneys.
- Angiotensin II Effects:
- Adrenal Glands: Stimulates aldosterone secretion, enhancing Na⁺ reabsorption and K⁺ excretion.
- Arterioles: Causes vasoconstriction.
- Pituitary Gland: Promotes ADH release, increasing water reabsorption.
- Sympathetic Nervous System: Increases activity.
Overall Outcome
- Blood Pressure Regulation: Increases via salt and water retention, enhancing vascular volume and juxtaglomerular perfusion.
- Intrarenal Angiotensin II: Augments Na⁺ reabsorption in the proximal tubule, while extrarenal renin and angiotensin II act in vascular endothelium, adrenal glands, and the brain.
Renal Blood Flow (RBF)
- Oxygen Consumption: Renal oxygen consumption is directly linked to blood flow.
- Cardiac Output Distribution: The kidneys receive 20–25% of cardiac output.
- Regional Distribution:
- 80% of RBF supplies cortical nephrons.
- 10–15% is directed to juxtamedullary nephrons.
- Oxygen Tension:
- Renal Cortex: High blood flow supports filtration, resulting in low oxygen extraction (oxygen tension ~50 mmHg).
- Renal Medulla: Requires low blood flow to sustain osmotic gradients; high metabolic activity (oxygen tension ~15 mmHg) increases ischemia risk.
- RBF Redistribution: Sympathetic stimulation, catecholamines, angiotensin II, and heart failure shift flow toward juxtamedullary nephrons.
- Functional Anatomy:
- A single renal artery from the aorta divides into interlobar, arcuate, and interlobular arteries.
- Blood enters glomeruli via afferent arterioles and exits through efferent arterioles, which drain into peritubular capillaries for reabsorption. Venous blood returns via the renal vein to the inferior vena cava
Renal Plasma Flow (RPF)
- Measurement: Assessed via p-aminohippurate (PAH) clearance, as PAH is entirely cleared from plasma in one renal passage.
- Normal Values:
- RPF: ~660 mL/min.
- RBF: ~1200 mL/min.
Glomerular Filtration Rate (GFR)
- Definition: The volume of plasma filtered into Bowman’s capsule per unit time (~20% of RPF).
- Measurement:
- Inulin Clearance: A precise GFR marker as inulin is filtered without reabsorption or secretion.
- Creatinine Clearance: Practical but overestimates GFR due to tubular secretion.
- Normal GFR: ~120 ± 25 mL/min (men), ~95 ± 20 mL/min (women).
- Filtration Fraction (FF): Ratio of GFR to RPF, typically ~20%.
- Regulation:
- Afferent arteriolar dilation or efferent arteriolar constriction increases FF and preserves GFR even with reduced RPF.
Control Mechanisms Regulating RBF
Intrinsic Regulation: Autoregulation of RBF
- Range: Maintained between a MAP of 80–180 mmHg via afferent arteriole myogenic responses to pressure changes.
- Outside Limits: RBF becomes pressure-dependent when MAP is below 50 mmHg, halting glomerular filtration.
Tubuloglomerular Feedback
- Function: Stabilizes GFR across a range of perfusion pressures.
- Mechanism:
- Increased GFR → Elevated tubular flow → Reflexive GFR reduction.
- Decreased GFR → Reduced tubular flow → Reflexive GFR increase.
- Involves macula densa and mesangial cells modulating afferent arteriole tone via calcium, renin, and adenosine signaling.
Hormonal Regulation
RAAS
- Triggers: Reduced afferent arteriolar pressure, increased sympathetic activity, or decreased distal sodium load.
- Effect: Angiotensin II constricts afferent and efferent arterioles, with potential GFR reduction at high levels.
Epinephrine and Norepinephrine
- Action: Constrict afferent arterioles but often preserve GFR through renin and angiotensin II-mediated compensatory mechanisms.
Aldosterone and Catecholamines
- Preservation of GFR: Mediated by angiotensin-induced prostaglandin synthesis, which NSAIDs can inhibit.
Prostaglandins
- Function: Vasodilatory prostaglandins (PGD2, PGE2, PGI2) safeguard renal perfusion during systemic hypotension and stress.
Atrial Natriuretic Peptide (ANP)
- Action: Promotes vasodilation and sodium excretion in response to atrial distension.
- Mechanism:
- Dilates afferent and constricts efferent arterioles.
- Relaxes mesangial cells, enhancing GFR.
Neuronal and Paracrine Regulation
- Sympathetic Innervation: Originates from T4–L1, affecting the renal plexuses.
- Sympathetic Activity:
- Reduces RBF via β1-adrenergic stimulation of juxtaglomerular cells and α1-mediated vasoconstriction.
- α1-Adrenergic receptors increase sodium reabsorption in proximal tubules.
- α2-Adrenergic receptors decrease sodium reabsorption and promote water excretion.
Dopamine and Fenoldopam
- Action: Induce afferent and efferent arteriolar dilation via D1-receptor activation, enhancing RBF and natriuresis.
Links
- Renal transplant
- Renal replacement therapy
- ICU and renal disease
- Anaesthesia and renal disease
- Vascular physiology
- Polytrauma and haemorrhagic shock
References:
- Allman K, Wilson I, O’Donnell A. Oxford Handbook of Anaesthesia. Vol. 4. Great Clarendon Street, Oxford, OX2 6DP, United Kingdom: Oxford University Press; 2016.
- Butterworth J, Mackey D, Wasnick J. Morgan and Mikhail’s Clinical Anesthesiology, 7th Edition. 7th edition. New York: McGraw Hill Medical; 2022.
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org/
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
Summaries:
Renal Physiology
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “214dd6dc-9383-4758-b441-681c10eda985”



