- Scoliosis
- Paediatric Scoliosis
- Links
{}
Scoliosis
Introduction
Definition
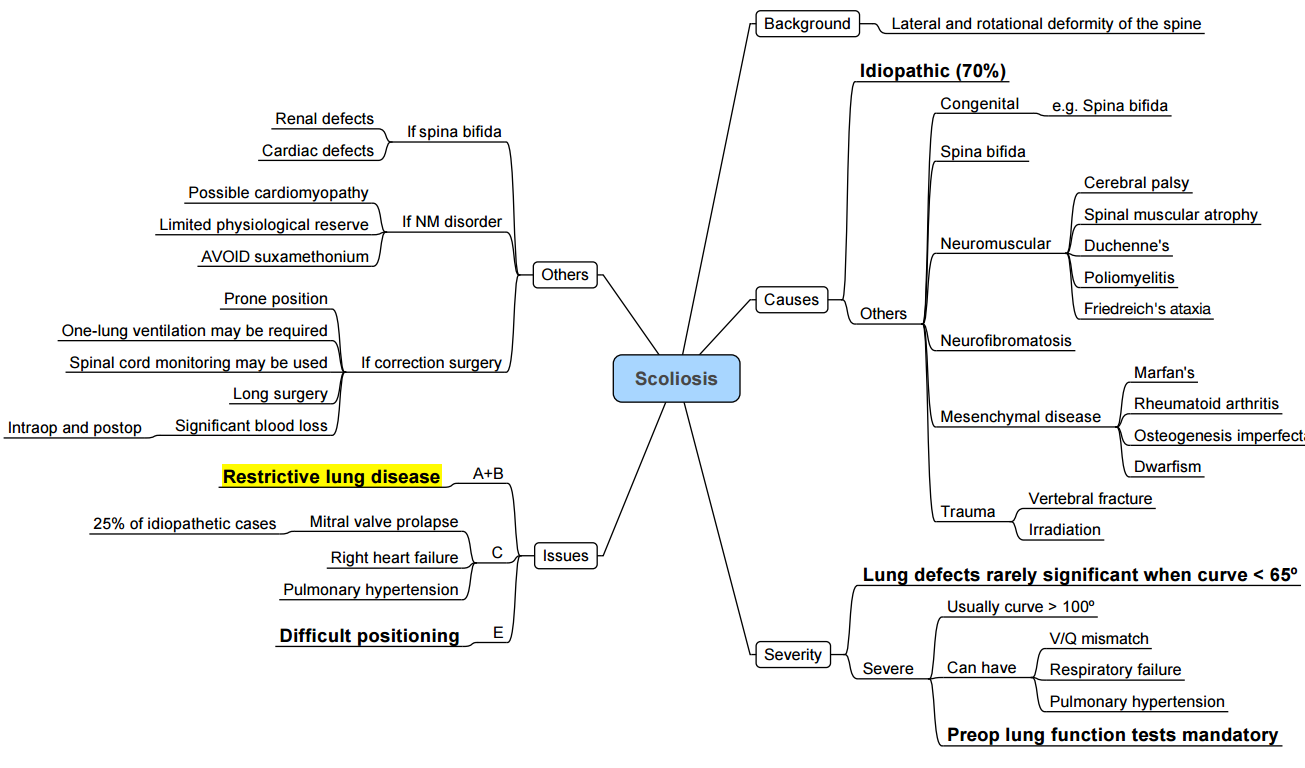
- Scoliosis is a three-dimensional deformity of the vertebral column characterised by a lateral curvature ≥ 10° measured by the Cobb method on an upright postero-anterior radiograph, usually accompanied by vertebral rotation.
- Kyphoscoliosis denotes combined coronal and sagittal deformity and confers greater pulmonary compromise.
Epidemiology & Natural History
- Adolescent idiopathic scoliosis (AIS) accounts for ~80 % of cases and has a female predominance (≈3:1). Curve progression risk increases with growth potential (Risser < 2, open triradiate cartilage) and Cobb angle > 30°.
- Early-onset scoliosis (< 10 yr) carries a higher incidence of restrictive lung disease and pulmonary hypertension in adulthood.
Aetiology & Classification
| Category | Examples | Key Anaesthetic Issues |
|---|---|---|
| Idiopathic • Infantile (< 3 yr) • Juvenile (3–10 yr) • Adolescent (10 yr–skeletal maturity) |
Usually otherwise healthy | Large blood loss, difficult positioning |
| Neuromuscular | Cerebral palsy, Duchenne muscular dystrophy (DMD), spinal muscular atrophy | Aspiration risk, cardiomyopathy (DMD), severe restrictive lung disease |
| Congenital | Hemivertebrae, failure of segmentation, spinal dysraphism | Associated renal & cardiac anomalies, difficult airway |
| Syndromic | Marfan syndrome, neurofibromatosis, osteogenesis imperfecta | Aortopathy, dural ectasia, brittle bones |
| Degenerative (adult) | Osteoarthritis, osteoporosis | Elderly physiology, anticoagulation |
| Other | Post-traumatic, infective (TB), neoplastic | Sepsis optimisation, metastatic disease |
Indications for Surgical Correction
- Progressive thoracic curve > 50 ° or lumbar curve > 40 ° despite bracing.
- Significant coronal or sagittal imbalance causing pain or cardiopulmonary impairment.
- Rapid progression (> 10 ° yr⁻¹) in skeletally immature patients.
Anaesthetic Challenges in Major Spinal Fusion
| Domain | Key Considerations |
|---|---|
| Respiratory | Restrictive pattern (↓FVC, ↓FEV₁). Cobb > 60 ° predicts impaired gas transfer; Cobb > 80 ° → nocturnal hypoventilation; Cobb > 100 ° → chronic hypercapnia & pulmonary hypertension. |
| Cardiovascular | Mitral valve prolapse (AIS), dilated cardiomyopathy (DMD), systemic hypertension (NF1). Echo ± cardiology review if symptomatic or neuromuscular disease. |
| Airway | Difficult intubation with cervical/thoracic curves; pre-op imaging if atlanto-axial instability suspected. |
| Positioning (prone) | Pressure injury, ocular perfusion (POVL), venous air embolism (VAE); use Jackson or Wilson frame to avoid abdominal compression. |
| Blood Loss | Multilevel osteotomies & muscle dissection → average 20–80 mL kg⁻¹; multimodal blood-conservation (high-dose tranexamic acid, cell salvage, controlled hypotension). |
| Neuro-monitoring | Somatosensory evoked potentials (SSEP) & transcranial motor evoked potentials (tcMEP); avoid long-acting neuromuscular blockers, maintain MAP ≥ 70 mmHg during instrumentation. |
| Pain & ERAS | Severe early nociceptive pain plus neuropathic component; multimodal opioid-sparing regimen improves mobilisation and length of stay. |
Pre-operative Assessment & Optimisation
History & Examination
- Exercise tolerance, orthopnoea, sleep-disordered breathing.
- Document baseline neuro-status and functional handgrip (wake-up test ability).
- Screen for difficult airway and syndromic features.
Investigations (minimum)
| Test | Thresholds warranting further work-up |
|---|---|
| Spirometry (FEV₁ & FVC) | FVC < 50 % predicted → anaesthesia + respiratory review |
| Chest radiograph | Measure Cobb; Cobb > 60 ° → arterial blood gas (ABG) & full pulmonary function tests (PFTs) |
| ABG | PaCO₂ > 45 mmHg → plan postoperative ventilation/ICU |
| Echocardiogram | All neuromuscular scoliosis or clinical signs of cardiomyopathy/pulmonary hypertension |
| Laboratory | FBC, coagulation, renal profile, type & screen (2 units on standby) |
Optimisation
- Respiratory: Treat infections; bronchodilators for reversible obstruction; nightly non-invasive ventilation (if chronic hypercapnia); chest physiotherapy & incentive spirometry.
- Cardiac: Optimise heart failure, beta-blockade or ACE-is; cardiology review for DMD.
- Nutrition: Aim albumin > 30 g L⁻¹; high-protein supplements.
- Haematology: Treat iron-deficiency; consider erythropoietin for Hb < 12 g dL⁻¹.
- Counselling: Explain neuro-monitoring, potential need for staged surgery, postoperative ventilation and analgesia plan.
Intra-operative Management
Monitoring & Access
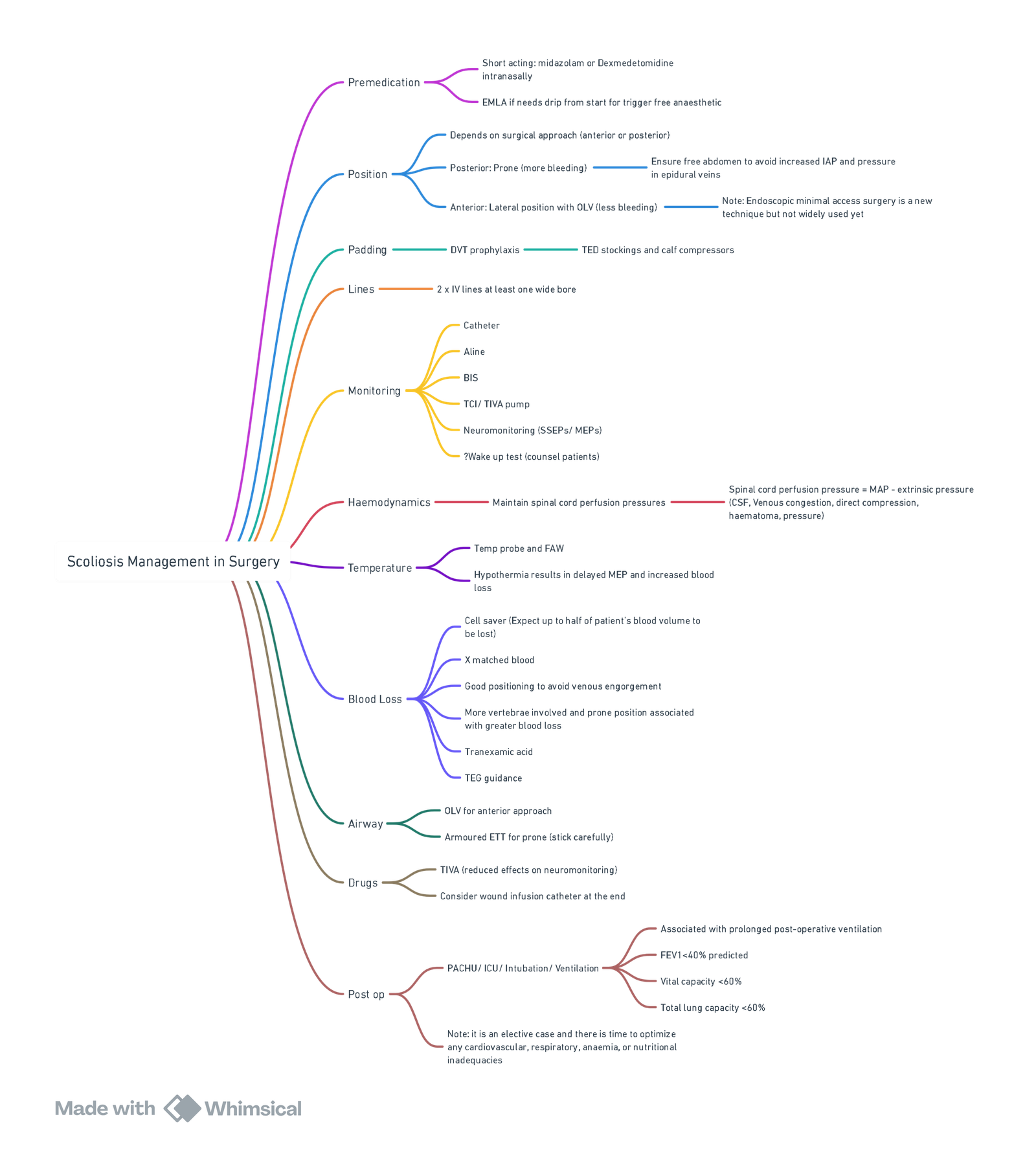
- Standard ASA monitors + invasive arterial pressure, large-bore peripheral IV × 2, ± central line if anticipated > 1 blood volume loss.
- Bispectral index (BIS) or processed EEG to titrate total intravenous anaesthesia (TIVA).
- Cell salvage with leucocyte filter; antifibrinolytic infusion
Anaesthetic Technique
| Aspect | Current Evidence-based Recommendation |
|---|---|
| Induction | Propofol (2–3 mg kg⁻¹) ± short-acting opioid; single intubating dose of rocuronium (0.6 mg kg⁻¹) is acceptable—reverse with sugammadex before baseline tcMEP; alternatives: no relaxant if difficult airway predicted. |
| Maintenance | TIVA (propofol 100-150 μg kg⁻¹ min⁻¹ + remifentanil 0.1–0.3 μg kg⁻¹ min⁻¹) minimises tcMEP suppression; inhalational ≤ 0.5 MAC with propofol infusion is acceptable. Dexmedetomidine 0.3–0.7 μg kg⁻¹ h⁻¹ reduces propofol dose and opioid requirements without clinically significant SEP changes. |
| Blood-sparing | Tranexamic acid 30 mg kg⁻¹ loading over 15 min, then 10 mg kg⁻¹ h⁻¹ (high-dose 100 mg kg⁻¹ + 10 mg kg⁻¹ h⁻¹ yields greatest reduction but uncertain safety—reserve for high-risk cases). |
| Controlled Hypotension | Target MAP 65–70 mmHg during exposure; raise to ≥ 70 mmHg for instrumentation/osteotomy and ≥ 80 mmHg for neuro-deficit or spinal cord injury. |
| Fluids | Balanced crystalloid 3–4 mL kg⁻¹ h⁻¹; albumin or viscoelastic-guided coagulation products to maintain ROTEM target values. |
| Temperature | Forced-air warming, fluid warmers; core T° > 36 °C to preserve evoked potentials. |
Positioning (Prone)
- Eyes free of pressure, head neutral, abdomen off table to limit venous engorgement.
- Padding of chest, iliac crests, knees and elbows; frequent checks after repositioning.
- Document eyes checks hourly to mitigate postoperative visual loss.
Neuro-monitoring Response Algorithm
| Event | Immediate Actions |
|---|---|
| tcMEP/SSEP amplitude ↓ > 50 % | ↑ MAP to ≥ 85 mmHg; normalise Hb & oxygenation; check anaesthetic depth; exclude mechanical causes (distraction rod, hypotension, hypothermia); consider wake-up test. |
Post-operative Care
Immediate (0–24 h)
- Extubate in theatre or ICU depending on lung function, blood loss and neuromuscular disease.
- Haemodynamic goals: MAP ≥ 70 mmHg (≥ 80 mmHg if intra-operative cord compromise).
- Neuro-checks: motor power & sensation q30 min (6 h) → q1 h (24 h).
- Analgesia: multimodal regimen
- Ketamine 0.25 mg kg⁻¹ h⁻¹ (24 h)
- Dexmedetomidine 0.3–0.5 μg kg⁻¹ h⁻¹ (sedation score ≤ -2)
- Acetaminophen 15 mg kg⁻¹ 6-hourly
- Reduced-dose opioid PCA (morphine 0.02 mg kg⁻¹ demand, lock-out 8 min)
- Erector spinae plane block (ESPB) with 0.375 % ropivacaine 20 mL side⁻¹ lowers early NRS and morphine use.
- VTE prophylaxis: intermittent pneumatic compression intra-op → LMWH 12–24 h post-op once haemostasis secured; continue until full ambulation.
Ongoing (Day 1–3)
- Transition to oral multimodal analgesia (paracetamol, tramadol/controlled-release oxycodone ± NSAID if surgeon agrees).
- Physiotherapy: sit-to-stand on Day 1, ambulation Day 2.
- Monitor ileus, urinary retention, wound drainage, SIADH (in neuromuscular disease).
Complications & Prevention
| Complication | Prevention & Early Detection |
|---|---|
| Massive blood loss | High-dose TXA, cell salvage, viscoelastic-guided transfusion |
| Hypothermia | Active warming, warmed fluids |
| POVL | Padding, keep head neutral, limit anaemia/hypotension |
| VTE | Early mobilisation, mechanical + LMWH prophylaxis |
| Airway oedema | Judicious fluids, leak test before extubation |
| Neurological injury | Continuous SSEP/tcMEP, maintain perfusion, prompt wake-up test |
| Chronic pain | Intra-operative ketamine & dexmedetomidine, ESPB, early physiotherapy |
Analgesic Techniques Summary
| Technique | Evidence & Caveats |
|---|---|
| ESPB | RCTs show ↓ opioid by ~30 % first 24 h; safe when performed pre-incision. |
| Intrathecal Morphine (3-5 μg kg⁻¹) | Excellent analgesia but respiratory monitoring ≥ 24 h. |
| Wound catheters | Simple; infection risk minimal with ≤ 48 h dwell. |
| Dexmedetomidine infusion | Useful adjunct but prolongs sedation at doses > 0.7 μg kg⁻¹ h⁻¹. |
| Ketamine infusion | Superior to dexmedetomidine for opioid-sparing; avoid in psychosis. |
| NSAIDs | Controversial effect on fusion; short-course ketorolac (≤ 72 h) appears safe. |
Risk-mitigation Checklist
- Two cross-matched units immediately available.
- TXA drawn-up before knife-to-skin.
- Neuromonitoring baseline established before instrumentation.
- Eye checks & pressure points logged hourly.
- Wake-up test protocol printed and available.
- Post-op ICU bed confirmed.
- Multidisciplinary huddle (surgeon, anaesthetist, neuro-physiologist, nursing) before induction.
View or edit this diagram in Whimsical.
Paediatric Scoliosis
Anaesthetic Concerns
(a) Respiratory
- Progressive restrictive pattern → ↓ forced vital capacity (FVC) & total lung capacity.
- FVC < 40 % predicted or Cobb > 90 ° strongly predicts need for postoperative ventilation.
- Spine surgery may transiently reduce PFTs by up to 60 % with recovery over 1–2 months.
- ↓ Chest-wall compliance, V/Q mismatch → chronic hypoxaemia.
- Hypercapnia (PaCO₂ > 45 mmHg) indicates advanced disease and correlates with pulmonary hypertension (PH).
(b) Cardiovascular
- Chronic hypoxia ± sleep-disordered breathing → PH → right-ventricular hypertrophy/failure.
- Up to 30 % of idiopathic cases have valvular abnormalities; congenital heart disease incidence ≈ 4 %. Cardiac lesions are more common in males and thoracolumbar curves.
Common Problems
| Domain | Key Points | Practical Implications |
|---|---|---|
| Respiratory | FVC & VC fall with curve severity; FVC < 40 % predicted → high likelihood of postoperative ventilation; lung volumes may fall another 40 % immediately post-fusion and normalise over 2 months. | Plan elective ICU, consider postoperative non-invasive ventilation; pre-op airway clearance. |
| Cardiovascular | ↑ pulmonary vascular resistance independent of curve size; vigilance for valvular lesions & congenital heart disease | Baseline ECG & echo; optimise PH/treat heart failure. |
| Neurological | Baseline deficits variable; risk of cord injury during correction. | Document exam; consent for wake-up test. |
| Neuromuscular aetiology | DMD & other myopathies → cardiomyopathy, respiratory weakness, anaesthesia-induced rhabdomyolysis (AIR) | Avoid succinylcholine & volatile agents; use TIVA; anticipate prolonged ventilation. |
| Nutrition | Chronic illness ± rapid growth may cause malnutrition. | Pre-op dietetic input; albumin > 30 g L⁻¹. |
Peri-operative Issues & Strategies
| Phase | Key Actions |
|---|---|
| Pre-operative | • Full cardiorespiratory work-up (PFT, ABG if Cobb > 60 ° or FVC < 50 %). • Echo for all neuromuscular curves or murmurs. • Optimise respiratory muscle training, treat infections, initiate nocturnal BiPAP if chronic hypercapnia. • Bloods: FBC, coagulation, group & screen (≥ 2 units ready). |
| Induction & Maintenance | • TIVA (propofol–remifentanil) ± adjunct dexmedetomidine 0.3–0.6 µg kg⁻¹ h⁻¹ preserves SEP/MEP. • One intubating dose of rocuronium acceptable; reverse with sugammadex before baseline tcMEP. • Neuromuscular disease: omit muscle relaxant, avoid volatile agents & succinylcholine (AIR risk). |
| Position (prone) | Jackson/Wilson frame; free abdomen; eye checks hourly. |
| Spinal cord protection | Continuous SSEP/tcMEP; maintain MAP ≥ 70 mmHg (≥ 80 mmHg if signal loss). |
| Blood conservation | TXA 30 mg kg⁻¹ load → 10 mg kg⁻¹ h⁻¹ infusion; cell salvage. |
| Analgesia | Multimodal regimen (see ERAS protocol); ESPB reduces 24-h opioid by ≈ 30 %. |
Enhanced Recovery After Surgery (ERAS): Red Cross War Memorial Children’s Hospital Pain Protocol
- The protocol below integrates recent RCT data on ketamine, gabapentin and ESPB to optimise opioid-sparing while maintaining compatibility with neuro-monitoring.
Pre-operative
| Team | Intervention |
|---|---|
| Orthopaedic | Gabapentin 15 mg kg⁻¹ PO night before & morning of surgery, then 8-hourly (target 10 mg kg⁻¹ q8 h). Pre-/post-fusion gabapentin halves early opioid use and pain scores. |
| Anaesthesia | Counsel patient/family; assess PCA suitability; provide information sheet. |
Intra-operative
| Step | Drug & Dose |
|---|---|
| Loading analgesics | Paracetamol 20 mg kg⁻¹ IV; morphine 0.1–0.2 mg kg⁻¹ IV (after baseline MEP). |
| Adjuvants (post-monitoring) | Ketamine infusion 0.25–0.35 mg kg⁻¹ h⁻¹ (supported by 2022 meta-analysis showing 25 % opioid reduction). Clonidine 1–2 µg kg⁻¹ IV or switch to dexmedetomidine 0.3–0.6 µg kg⁻¹ h⁻¹. |
| Regional | Bilateral ultrasound-guided ESPB before closure: bupivacaine 3 mg kg⁻¹ total (0.5 mL kg⁻¹ side⁻¹) + clonidine 1 µg kg⁻¹. |
| PONV prophylaxis | Dexamethasone 0.15 mg kg⁻¹ IV + ondansetron 0.15 mg kg⁻¹ IV. |
Post-operative (ICU Day 0)
| Medication | Schedule |
|---|---|
| Paracetamol | 15 mg kg⁻¹ IV q6 h. |
| Clonidine | 1–3 µg kg⁻¹ PO q8 h or dexmedetomidine 0.3–0.5 µg kg⁻¹ h⁻¹ (avoid Ramsay > 4). |
| Gabapentin | Continue 3–5 mg kg⁻¹ PO q8 h → titrate to target 10 mg kg⁻¹ q8 h. |
| Ketamine | 0.25–0.35 mg kg⁻¹ h⁻¹ for 24 h, then wean. |
| Morphine | PCA: background 0–0.5 mL h⁻¹; bolus 20 µg kg⁻¹; lock-out 5 min. |
| PONV | Ondansetron 0.15 mg kg⁻¹ IV q8 h. |
| Others | Lactulose 0.5 mL kg⁻¹ PO q12 h; early physiotherapy & caregiver distraction techniques. |
Ward Days 1–3
- Pain service: taper PCA, introduce ibuprofen 10 mg kg⁻¹ q8 h once haemostasis & renal function normal.
- Physiotherapy: edge-of-bed mobilisation Day 1; standing & walking Day 2 (coordinate timing with oral analgesic dosing)
Discharge (≈ Day 3–5)
| Drug | Dose & Duration |
|---|---|
| Paracetamol | 15 mg kg⁻¹ PO q6 h. |
| Ibuprofen | 10 mg kg⁻¹ PO q8 h. |
| Gabapentin | 10 mg kg⁻¹ PO q8 h × 2 weeks, then taper. |
| Tramadol (prn) | 1–2 mg kg⁻¹ PO q6 h. |
Key Points for Muscular Dystrophy
- Avoid succinylcholine and minimise/omit volatile agents–high risk of AIR with hyperkalaemic arrest.
- Use non-depolarising relaxants titrated to nerve stimulator; reverse with sugammadex 2 mg kg⁻¹.
- Post-operative ventilation likely when FVC < 40 % predicted or cardiomyopathy present; plan elective ICU.
Links
References:
- Hudec J, Prokopová T, Kosinová M, Gál R. Anesthesia and Perioperative Management for Surgical Correction of Neuromuscular Scoliosis in Children: A Narrative Review. J Clin Med. 2023 May 24;12(11):3651. doi: 10.3390/jcm12113651. PMID: 37297846; PMCID: PMC10253354.
- Fung, A. and Wong, P. C. (2023). Anaesthesia for scoliosis surgery. Anaesthesia &Amp; Intensive Care Medicine, 24(12), 744-750. https://doi.org/10.1016/j.mpaic.2023.09.004
- Anaesthetic Guideline for Posterior Approach to Scoliosis Corrective Surgery. UCT guideline
- Young, C. D., McLuckie, D., & Spencer, A. O. (2019). Anaesthetic care for surgical management of adolescent idiopathic scoliosis. BJA Education, 19(7), 232-237. https://doi.org/10.1016/j.bjae.2019.03.005
- Young CD, McLuckie D, Spencer AO. Anaesthetic care for surgical management of adolescent idiopathic scoliosis. BJA Education. 2019;19(7):232-237. pmc.ncbi.nlm.nih.gov
- Hudec J, Prokopová T, Kosinová M, Gál R. Anesthesia and perioperative management for surgical correction of neuromuscular scoliosis in children: a narrative review. J Clin Med. 2023;12(11):3651. pmc.ncbi.nlm.nih.gov
- Li X et al. The optimal dose of intravenous tranexamic acid for reducing blood loss in multilevel spine surgery: a network meta-analysis. BMC Musculoskelet Disord. 2025;26:8233. bmcmusculoskeletdisord.biomedcentral.com
- Madani S et al. Safety and efficacy of tranexamic acid in spinal surgery: a systematic review and meta-analysis. Cureus. 2025;17:e11165496. pmc.ncbi.nlm.nih.gov
- Xu H et al. Effects of dexmedetomidine on evoked potentials in spine surgery: a randomised controlled trial. BMC Anesthesiol. 2023;23:1990. bmcanesthesiol.biomedcentral.com
- Wang Y et al. Bilateral ultrasound-guided erector spinae plane block improves postoperative analgesia after posterior spinal fusion in paediatric idiopathic scoliosis: a randomised controlled trial. Eur J Anaesthesiol. 2024;41:123-131. pubmed.ncbi.nlm.nih.gov
- Wong A et al. Venous thromboembolism prophylaxis in elective spine surgery: a narrative review. Global Spine J. 2021;11(8):1214-1223. pmc.ncbi.nlm.nih.gov
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org/
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
- Yuan N, et al. Spinal fusion pulmonary management pathway. Johns Hopkins Medicine; 2023. hopkinsmedicine.org
- Elsamadicy N, et al. Postoperative pulmonary complications in complex paediatric spine surgery. Pediatr Pulmonol. 2021. pmc.ncbi.nlm.nih.gov
- Anderson DE, et al. Multimodal pain control with gabapentin in adolescent posterior spinal fusion: RCT. Spine Deform. 2020;8:177-185. pubmed.ncbi.nlm.nih.gov
- Mariscal G, et al. Ketamine for postoperative pain in AIS: meta-analysis. Eur Spine J. 2022;31:3492-3499. pubmed.ncbi.nlm.nih.gov
- Gao J, et al. Bilateral ESPB for paediatric posterior fusion: RCT protocol. Trials. 2024;25:498. pmc.ncbi.nlm.nih.gov
- Yang X, et al. Cardiac abnormalities in idiopathic scoliosis: risk-factor analysis. Sci Rep. 2025;15:16013. nature.com
- van den Bersselaar L, et al. Anaesthetic management in neuromuscular disease. Front Anesthesiol. 2023. pmc.ncbi.nlm.nih.gov
Summaries:
Paediatric scoliosis
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “54f8b1be-f289-4c8a-a70d-9d2f1e0af0ab”



