- Introduction and Epidemiology
- Pathogenesis
- Risk Factors
- Treatment of Postoperative Atrial Fibrillation (POAF)
- Anaesthesia for AF
- Complications of AF
{}
Introduction and Epidemiology
Acute AF
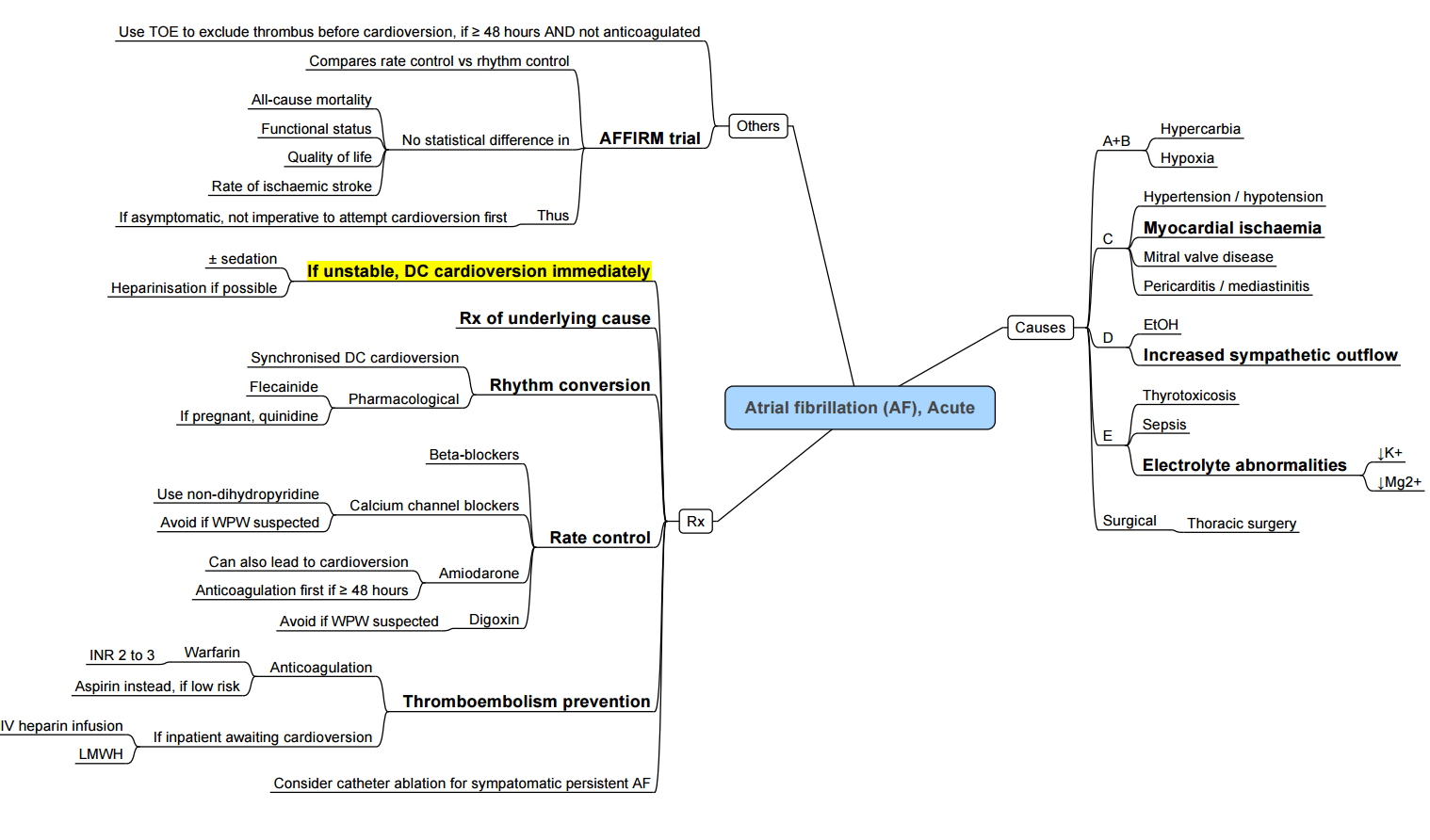
Chronic AF
Pathogenesis
| Pathogenesis | Mainly “Structural” Effects | Mainly “Electrical” Effects |
|---|---|---|
| Non-modifiable Factors | – Increasing age – Male gender |
– Channelopathies (altered ion channel function, e.g., Brugada, Long QT) – Familial & genetic factors – Lone (idiopathic) AF |
| Modifiable Factors | – Hypertension – Hyperthyroidism – Diabetes – Obesity – Obstructive sleep apnea – Excessive alcohol intake |
– Autonomic imbalance (increased sympathetic drive or high vagal tone) |
| Other Structural Factors | – Valvular heart disease (including rheumatic heart disease) – Heart Failure (systolic and/or diastolic dysfunction, including post-MI) – Cardiomyopathies (including hypertrophic, restrictive & dilated CM) – Congenital heart disease – Myocarditis – Pulmonary disease – Post-surgical state (especially after cardiac surgery) – Endurance athletic training |
Pathophysiological Mechanisms
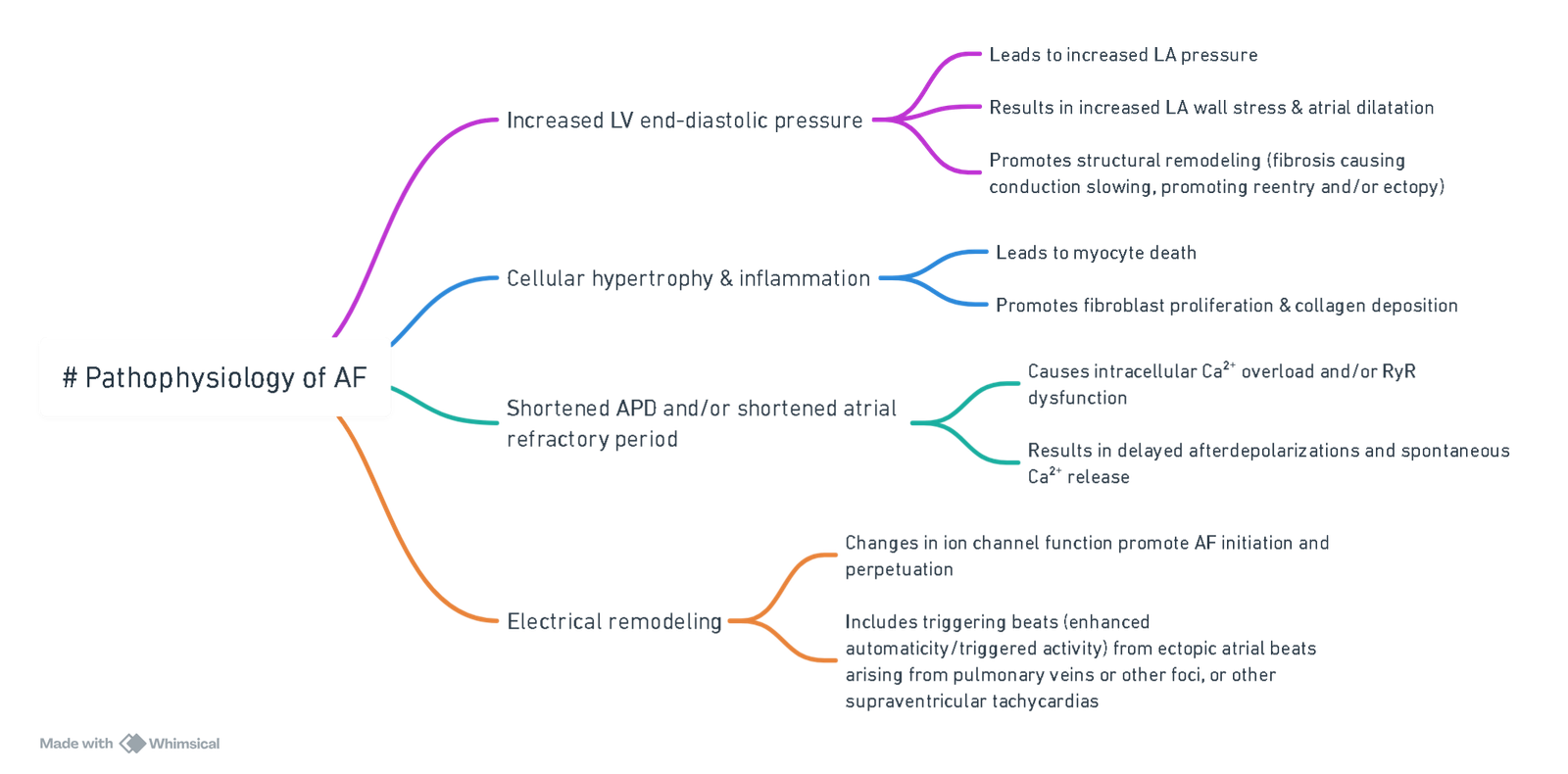
View or edit this diagram in Whimsical.
AF Classification
- Atrial fibrillation is the most common sustained arrhythmia, increasing in prevalence and incidence
- Paroxysmal AF: Terminates spontaneously within 7 days of onset
- Persistent AF: Lasts >7 days and/or requires cardioversion to restore sinus rhythm (SR)
- Permanent AF: Persistent AF, no longer pursuing rhythm control strategies
- Perioperative AF (POAF)
- 1%: minor surgery
- 5-10%: vascular or large colorectal surgery
- New-onset POAF occurs principally after thoracic and cardiac surgery
- Very low after exploratory thoracotomy or minimal resections of the lung, but higher after larger lung resections or esophagogastrectomy (10–30%)
- The incidence of POAF after cardiac surgery remains high: 30% after isolated coronary artery bypass grafting (CABG) surgery, 40% after valve surgery, and 50% after combined procedures.
- 15% for TAVI
- The time course of POAF (70% in the first 4 postoperative days) highlights the importance of temporary surgery-induced factors such as inflammation, sympathetic stimulation (probably the most relevant). and oxidative stress
- Atrial fibrillation increases the risk of stroke fivefold
- Increased hemodynamic instability, increased thromboembolic risk, and the management of OAC with or without heparin bridging
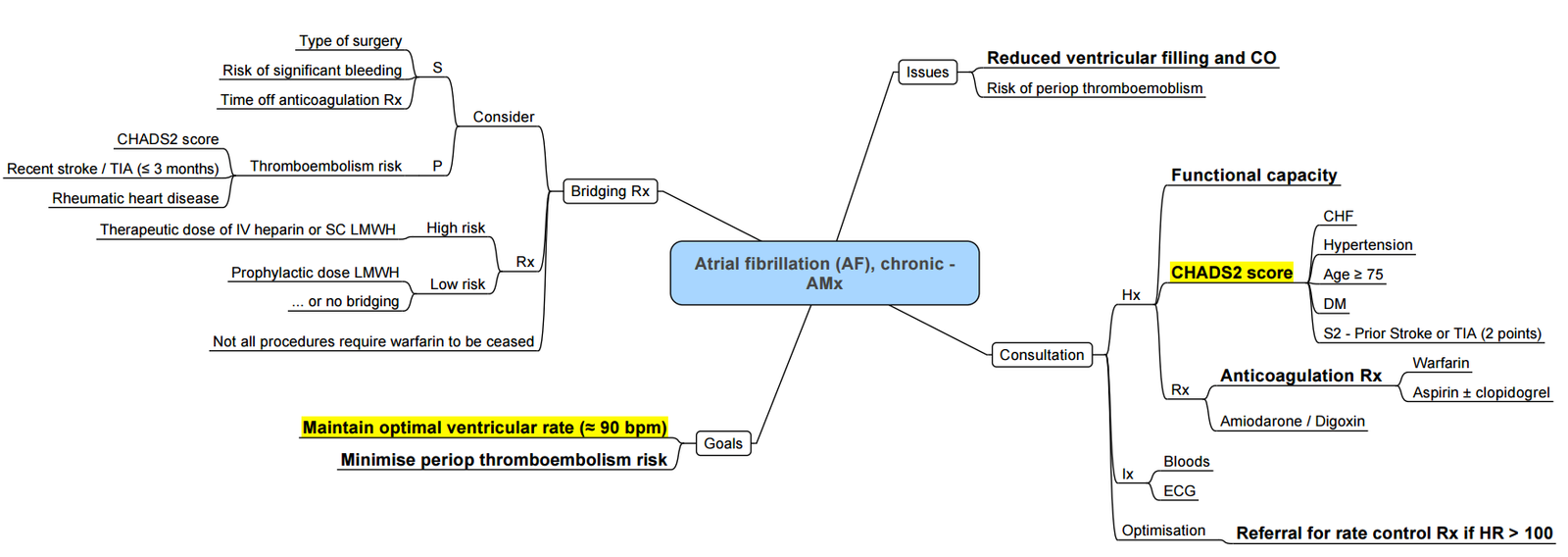
Risk Factors
Risk Factors for AF
- Traditional: age, hypertension, male sex, diabetes, valvular heart disease, heart failure, coronary heart disease, cardiac surgery, hyperthyroidism
- Emerging: obesity, chronic kidney disease, sleep apnea, excess alcohol consumption, genetic factors
Risk Factors for Perioperative AF (POAF)
Patient
- Advancing age, left atrial enlargement, obesity, previous history of AF, male gender, hypertension, COPD, heart failure, withdrawal of β-blockers, genetic factors
Perioperative Factors for POAF
- Surgical (atrial suture or ischemia)
- Inflammation, oxidative stress
- Sympathetic stimulation
- Acute volume changes
Triggering Factors for POAF
- Electrolyte imbalance
- Atrial premature contraction
- Enhanced adrenergic or vagal stimulation
Risk Stratification for Bleeding
The HAS-BLED score is a clinical tool used to estimate the 1-year risk of major bleeding in patients with atrial fibrillation (AF), particularly those on anticoagulation therapy (e.g., warfarin, DOACs). It helps guide decisions about initiating or continuing anticoagulation
HAS-BLED Acronym Breakdown (Score Range: 0–9)
| Letter | Risk Factor | Points |
|---|---|---|
| H | Hypertension (SBP >160 mmHg) | 1 |
| A | Abnormal liver or renal function (1 point each) | 1 or 2 |
| S | Stroke history | 1 |
| B | Bleeding history or predisposition | 1 |
| L | Labile INRs (time in therapeutic range <60%) | 1 |
| E | Elderly (>65 years) | 1 |
| D | Drugs (antiplatelets/NSAIDs) or alcohol use (1 point each) | 1 or 2 |
Scoring And Interpretation
- 0–1: Low risk
- 2: Moderate risk
- ≥3: High risk → requires caution and regular review of bleeding risk
Clinical Use
- A high HAS-BLED score does not contraindicate anticoagulation but prompts efforts to correct modifiable risk factors (e.g., uncontrolled hypertension, alcohol use, concomitant NSAIDs).
- Used alongside CHA₂DS₂-VASc to balance stroke vs bleeding risk.
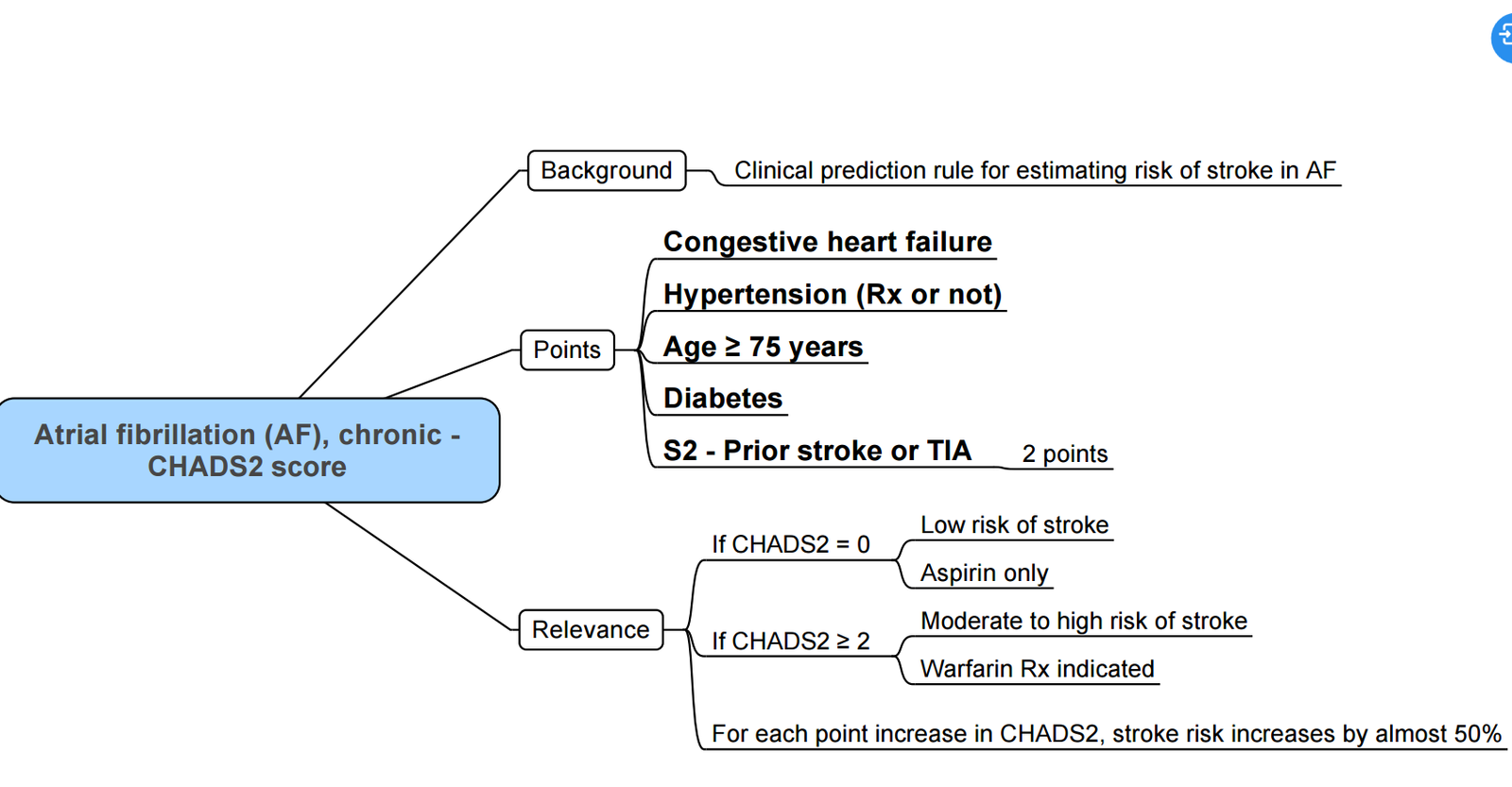
Risk for CVA
CHADS2 Score
Treatment of Postoperative Atrial Fibrillation (POAF)
Management Steps
-
Management of Co-existing Medical Conditions
- Identify and treat any underlying medical conditions contributing to POAF.
-
Cardioversion for Hemodynamically Unstable Patients
- Electrical cardioversion is recommended for patients who are hemodynamically unstable.
-
Rate-Control Strategy for Stable Patients
- For hemodynamically stable patients, a rate-control strategy is often first-line.
- Atrioventricular nodal-blocking agents such as beta-blockers, or nondihydropyridine calcium-channel antagonists (e.g., diltiazem, verapamil) are preferred.
- Amiodarone can be used to control heart rate independently of its rhythm-conversion effects.
- For hemodynamically stable patients, a rate-control strategy is often first-line.
-
Rhythm-Control Strategy
- If atrial fibrillation (AF) does not convert to sinus rhythm within 24 hours, a rhythm-control strategy should be pursued, with concurrent anticoagulation.
- Cardioversion is indicated for patients with a definitive AF onset of less than 48 hours, especially if they have risk factors for stroke.
Note
- POAF is often transient and self-limiting. Many patients, particularly those who have undergone non-cardiac surgery, may not require antiarrhythmic drugs (AAD) or anticoagulation therapy.
Prevention of POAF
- Beta-Blockers Continuation
- Continuing beta-blockers is recommended.
- Combination Therapy Evaluation
- The impact of a combination regimen including beta-blockers, amiodarone, statins, magnesium, and corticosteroids is still under evaluation.
Anticoagulation Management
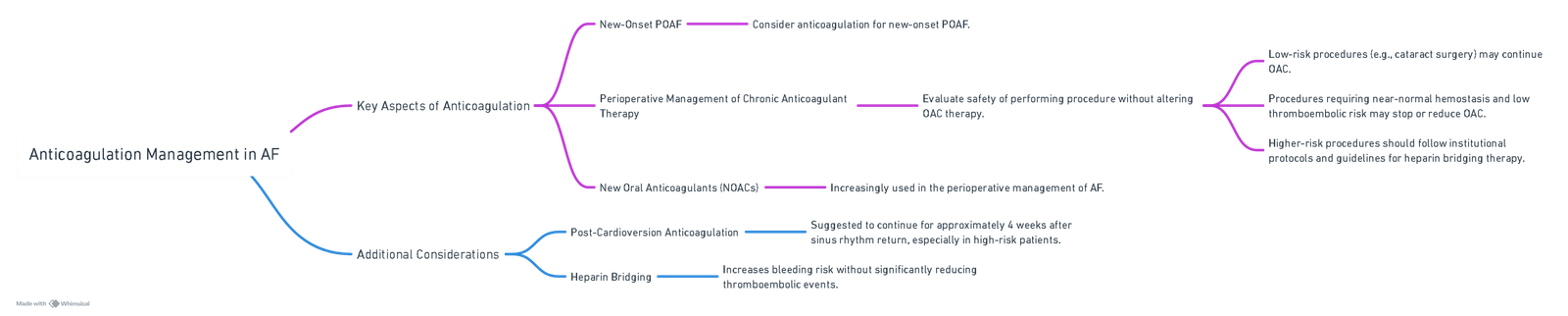
View or edit this diagram in Whimsical.
Vit K Antagonist (warfarin) for AF Requiring Invasive Procedures
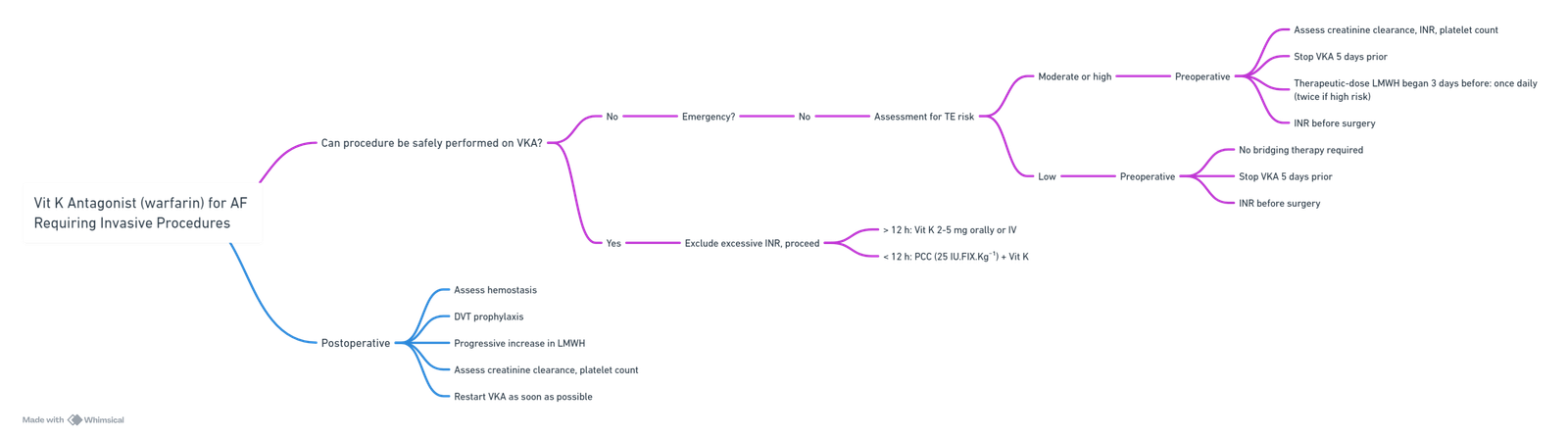
View or edit this diagram in Whimsical.
Management of Patients on NOAC
- Current guidelines for bridging NOAC before major surgery are cautious
- European and French societies recommend a schema similar to VKA management, with an interruption of oral therapy for 4 days before surgery and heparin bridging if necessary
- (1) Medically manage atrial fibrillation and continue anticoagulation in patients with atrial fibrillation for minor surgeries or those in which high blood loss is unlikely. Discontinue anticoagulation in surgical patients at high risk of bleeding (with appropriate bridging strategies as indicated), but resume as soon as the risk of surgical bleeding is considered to be low (Opinion-based evidence, Category A).
- (2) There is no evidence to suggest that continuation of aspirin in patients at risk for vascular complications reduces the risk of stroke after noncardiac surgery (Category A, Level 3).
Anaesthesia for AF
Perioperative Considerations
Preoperative
- Assessment of Underlying Causes:
- Cardiac: ischaemic heart disease, valvular pathology, heart failure.
- Pulmonary: pulmonary embolism, chronic obstructive pulmonary disease.
- Endocrine/metabolic: thyrotoxicosis, electrolyte disturbances (e.g., hypokalaemia, hypomagnesaemia).
- Drug toxicity (e.g., theophyllines, sympathomimetics).
- Time permitting, obtain cardiology input for new-onset AF or poor rate control (ventricular rate >100 bpm despite therapy).
- Rate Control:
- Continue AV nodal blocking agents (β‑blockers or non‑dihydropyridine calcium channel blockers) until the day of surgery
- In new-onset or uncontrolled AF (resting rate >100 bpm despite optimisation), consider delaying elective procedures.
- Initial acute control: IV diltiazem (0.25 mg kg⁻¹ over 2 minutes) or IV metoprolol (2.5–5 mg increments).
- Anticoagulation Strategy:
- Employ CHADS₂‑VASc and HAS‑BLED for stroke versus bleeding risk stratification.
- For low-risk patients on direct oral anticoagulants (DOACs), cessation 24 hours preoperatively without bridging is typically safe.
- If bridging indicated:
- UFH infusion: stop 4–6 hours preoperatively.
- LMWH therapeutic dose: stop 24 hours preoperatively.
- Liaise with haematology or cardiology for complex cases.
Intraoperative
- Avoid and Correct Triggers:
- Maintain normovolaemia; avoid hypo‑ or hypervolaemia.
- Monitor and correct electrolytes (maintain K⁺ >3.5 mmol L⁻¹, Mg²⁺ 0.7–1.0 mmol L⁻¹).
- Prevent hypoxia and hypotension.
- Avoid desflurane due to sympathetic stimulation and pro‑arrhythmic effects.
- Management of Atrial Fibrillation:
- Unstable AF (haemodynamic compromise): synchronised DC cardioversion.
- Stable AF:
- IV β‑blockers (esmolol infusion 25–300 µg kg⁻¹ min⁻¹) preferred.
- Non‑dihydropyridine calcium channel blockers (diltiazem infusion 5–10 mg h⁻¹) as alternative.
- Amiodarone (150 mg IV over 10 minutes then 1 mg min⁻¹ for 6 hours) for refractory cases.
- Transoesophageal echocardiography if suspicion of intracardiac thrombus or structural pathology.
Postoperative
- Monitoring and Investigations:
- Continuous ECG monitoring in high‑risk or unstable patients.
- Troponin assay if ischaemia suspected.
- Chest X‑ray for pulmonary complications.
- Point‑of‑care ultrasound (POCUS) to assess volume status and cardiac function if haemodynamic instability.
- Therapeutic Measures:
- Continue rate control therapy; restart baseline β‑blockers or calcium channel blockers.
- Treat unstable AF with DC cardioversion.
- For stable AF, maintain rate control as per intraoperative strategy.
- Resume or initiate antithrombotic therapy based on stroke/bleeding risk; involve cardiology for DOAC timing.
- Consider CPAP for patients with coexisting obstructive sleep apnoea.
- Disposition:
- Continue rate control agents for 1–6 weeks postoperatively with outpatient cardiology review.
- Provide clear instructions on anticoagulation management and follow‑up plan.
Complications of AF
- Disorganized atrial depolarizations without effective atrial contraction
Pathophysiology
- Ineffective atrial contraction
- Blood stasis in left atrium and atrial appendage
- Atrial clot formation and embolization
- Brain: TIA/stroke, dementia
- Kidneys: renal artery occlusion
- Gut: mesenteric ischemia
- Peripheral arteries: acute arterial occlusion
- Irregular, rapid ventricular response rate
- ↓ Diastolic filling time
- ↓ Preload (↓ LVEDV)
- Decompensated Frank-Starling response
- ↓ Stroke volume (SV)
- ↓ Cardiac output (CO)
- Pre-existing coronary, myocardial, or valvular disease
- Myocardial ischemia and infarction
- Tachycardia-induced cardiac remodeling and dilatation
- Tachycardia-induced cardiomyopathy
- Heart failure
Risk Factors
- Previous stroke
- Mitral disease
- Age >65
- Hypertension
- Diabetes
- Heart failure
- Female sex
- Vascular disease
Links
Past Exam Questions
Post-Operative Atrial Fibrillation in a Patient with Bullous Lung Disease
A 55-year-old man with bullous lung disease underwent an elective thoracotomy for a lobectomy. Four hours post-operatively, he develops fast atrial fibrillation with hypotension.
a) List 3 possible causes for the atrial fibrillation in this scenario. (3)
b) Briefly provide a description of appropriate management strategies in treating atrial fibrillation and then suggest the most appropriate strategy for this patient. (7)
Perioperative Coagulation Management in a Patient with a Subdural Haematoma
A 65-year-old patient with non-valvular atrial fibrillation on warfarin presents with a large subdural haematoma with a deteriorating level of consciousness. Her INR is found to be 9.
a) How will you manage this patient’s coagulation perioperatively? (6)
b) What would your approach be to re-institution of anticoagulation in this patient? (4)
References:
- Philip, Ivan; Berroëta, Clarisse; Leblanc, Isabelle. Perioperative challenges of atrial fibrillation. Current Opinion in Anaesthesiology 27(3):p 344-352, June 2014. | DOI: 10.1097/ACO.0000000000000070
- Karamchandani, K., Khanna, A. K., Bose, S., Fernando, R. J., & Walkey, A. J. (2020). Atrial fibrillation: current evidence and management strategies during the perioperative period. Anesthesia &Amp; Analgesia, 130(1), 2-13. https://doi.org/10.1213/ane.0000000000004474
- Liao, H., Poon, K. S., & Chen, K. (2013). Atrial fibrillation: an anesthesiologist’s perspective. Acta Anaesthesiologica Taiwanica, 51(1), 34-36. https://doi.org/10.1016/j.aat.2013.03.010
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 Atrial Fibrillation Guideline. Circulation. 2019;140(2):e125–e151.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation. Eur Heart J. 2021;42(5):373–498
- Kirchhof P, Fabritz L, Franz MR, et al. Management of postoperative atrial fibrillation: a European Heart Rhythm Association expert consensus. Europace. 2016;18(11):1602–1618.
- Wynn GJ, Todd DM, Webber MJ, et al. Acute management of atrial fibrillation: evolving evidence-based approaches. Br J Anaesth. 2018;120(3):501–511.
- Hindricks G, Potpara T, Boriani G, et al. 2022 EHRA consensus on perioperative management of anticoagulants. Europace. 2022;24(12):1764–1782.
- Gallagher MM, Prendergast BD. Perioperative management of anticoagulation in atrial fibrillation patients. Curr Opin Anaesthesiol. 2020;33(5):621–628.
- Chiang CE, et al. Pharmacological rate control in atrial fibrillation. J Am Coll Cardiol. 2019;74(15):1953–1965.
- Kotecha D, Holmes SR. Optimising rate control in atrial fibrillation: beta-blockers vs calcium channel blockers. Int J Cardiol. 2019;281:90–98.
- The Calgary Guide to Understanding Disease. (2024). Retrieved June 5, 2024, from https://calgaryguide.ucalgary.ca/
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org/
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
Summaries:
AF
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “6530b527-5def-4f12-83d6-7163a823edb8”



