- Physiology and Anatomy Summary
- Approach to Chronic Liver Disease
- Conduct of Anaesthesia for End-Stage Liver Disease (ESLD)
- Links
{}
Physiology and Anatomy Summary
(See ICU and liver disease for more comprehensive overview)
Anatomy
- Lobes & Segments: Divided into right and left lobes by the falciform ligament, with eight segments (Couinaud classification) based on vascular supply.
- Vascular Supply: Dual blood supply–hepatic artery (25-50%) (oxygen-rich) and portal vein (50-75%) (nutrient-rich but deoxygenated). Hepatic veins drain into the IVC.
- Biliary System: Bile is secreted by hepatocytes, drains into bile canaliculi, then the common hepatic duct, and joins the cystic duct from the gallbladder to form the common bile duct, which empties into the duodenum via the ampulla of Vater.
- Functional Units: The hepatic lobule contains hepatocytes arranged around a central vein, with sinusoids allowing exchange between blood and liver cells.
Physiology
- Metabolism
- Carbohydrates: Glycogen storage, gluconeogenesis, glycolysis.
- Proteins: Synthesis of albumin, clotting factors (except VIII), urea cycle (ammonia detoxification).
- Lipids: Lipoprotein metabolism, cholesterol and bile acid synthesis.
- Drug Metabolism (Biotransformation)
- Phase I (Oxidation, reduction, hydrolysis)–Cytochrome P450 enzymes.
- Phase II (Conjugation)–Increases solubility for renal/biliary excretion.
- Hemostasis
- Produces clotting factors (I, II, V, VII, IX, X, XI, XIII) and anticoagulants (Protein C, S, antithrombin III).
- Clearance of activated clotting factors; dysfunction leads to coagulopathy (↑ INR, ↓ fibrinogen, thrombocytopenia from hypersplenism).
- Volume Regulation
- Synthesizes albumin (oncotic pressure maintenance).
- Acts as a blood reservoir (~500 mL can be mobilized).
- Immune Function
- Kupffer cells in sinusoids clear bacteria and endotoxins.
- Synthesizes acute phase proteins (CRP, complement).
- Bile Production & Excretion
- Bile salts emulsify fats for digestion.
- Bilirubin metabolism: Unconjugated bilirubin → conjugated in liver → excreted in bile.
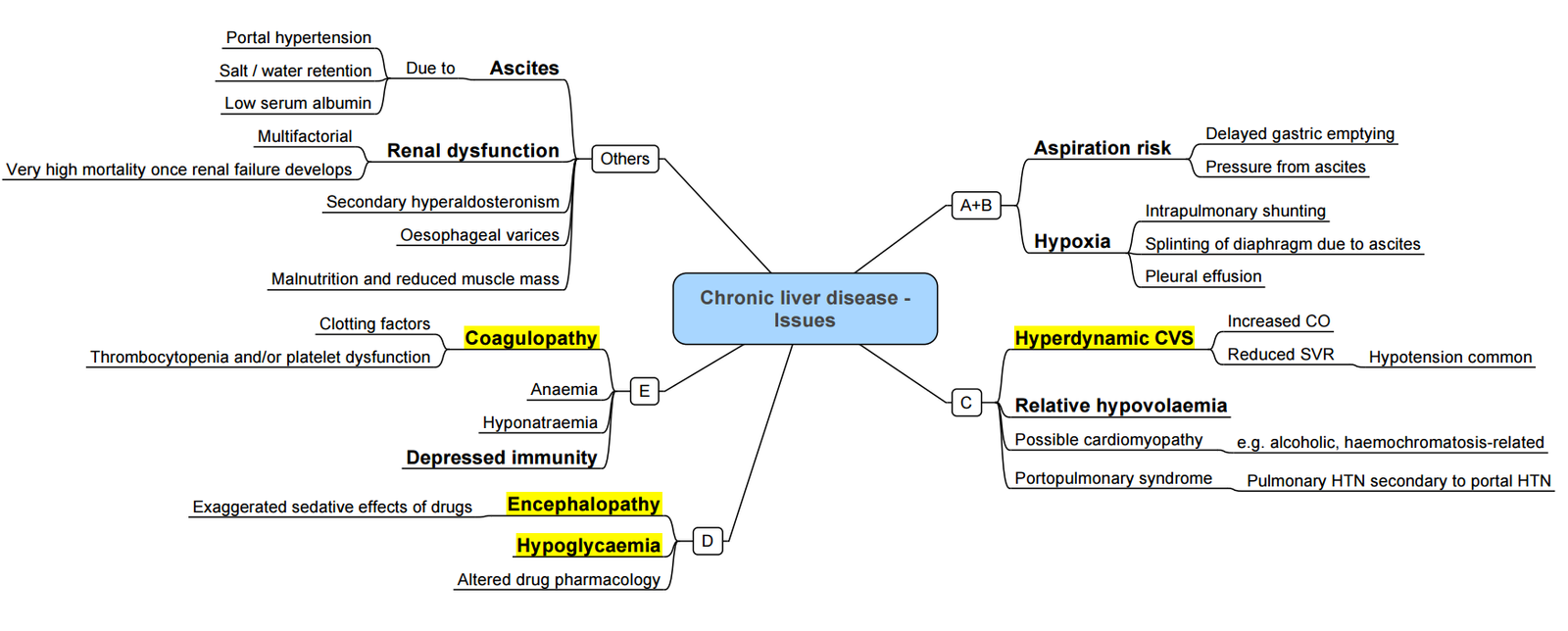
Approach to Chronic Liver Disease
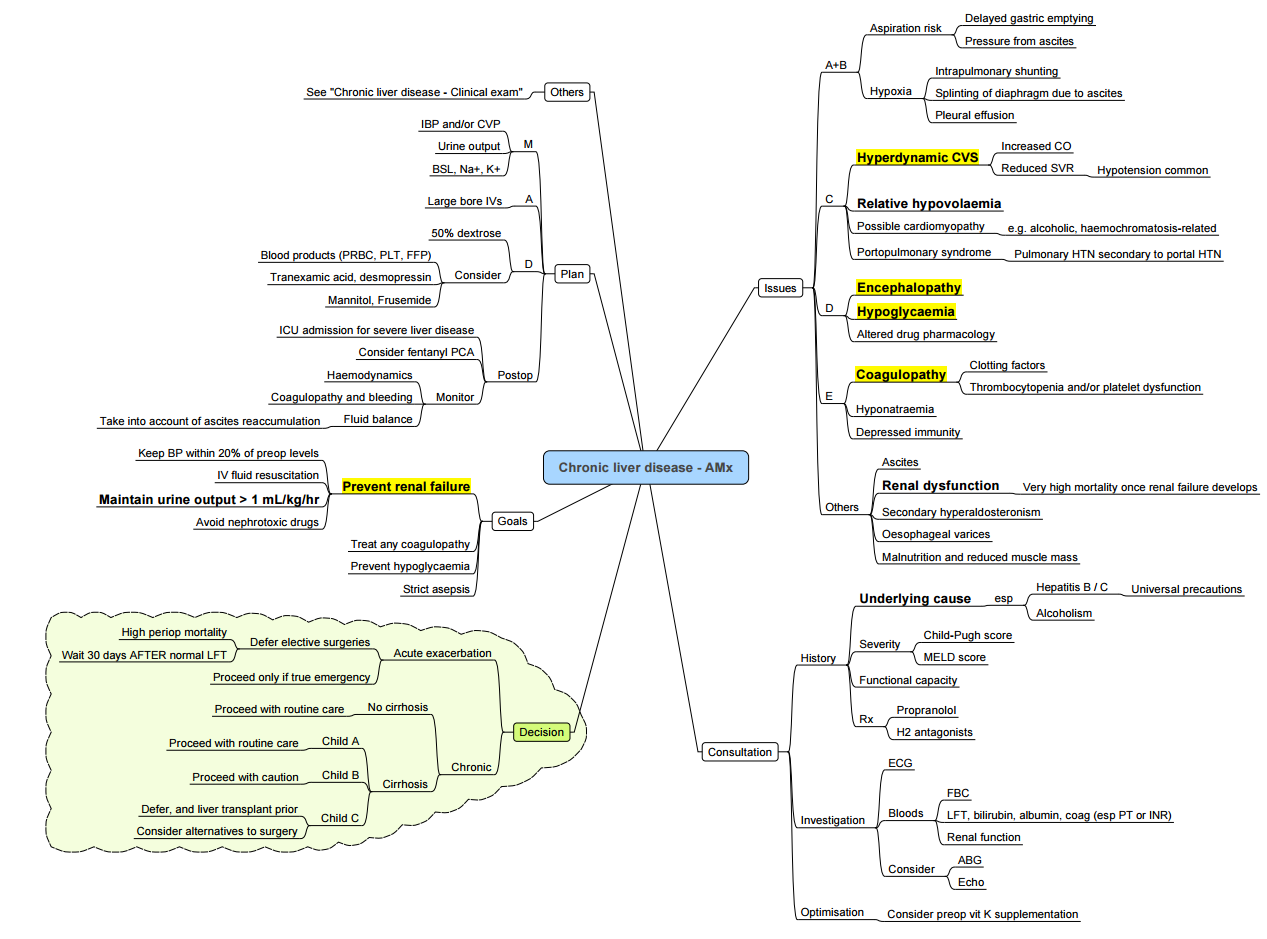
Pathophysiology of Decompensated Liver Cirrhosis
Precipitants for Decompensation
- Progressive liver disease
- Hypotension
- Infections
- Hepatocellular carcinoma
- Portal vein thrombosis
Pathway from Stable Compensated to Decompensated Liver Cirrhosis
- Stable Compensated Liver Cirrhosis → Decompensated Liver Cirrhosis:
- Synthetic Dysfunction:
- Infection → Coagulopathy
- Immune Dysfunction
- Reduced Toxin Removal:
- Jaundice
- Hepatic Encephalopathy
- Cardiovascular Dysfunction:
- Hepatorenal Syndrome
- Portal Hypertension:
- Varices → Variceal Haemorrhage
- Ascites
- Spontaneous Bacterial Peritonitis
- Synthetic Dysfunction:
Cardiovascular Complications in Liver Cirrhosis
Increased Levels of Vasodilating Substances
- Nitric Oxide
- Carbon Monoxide
- Prostacyclin
- Endocannabinoids
- Endothelium-derived Hyperpolarizing Factor
- Tumour Necrosis Factor-α
- Adrenomedullin
- Hydrogen Sulfide
Pathophysiological Pathway
- Liver Dysfunction/Portal Hypertension →
- Increased Levels of Vasodilating Substances →
- Splanchnic Vasodilation
- Systemic Vasodilation
- Bacterial Translocation and Inflammation →
- Increased Plasma Volume
- Increased Cardiac Output
- Increased Levels of Vasodilating Substances →
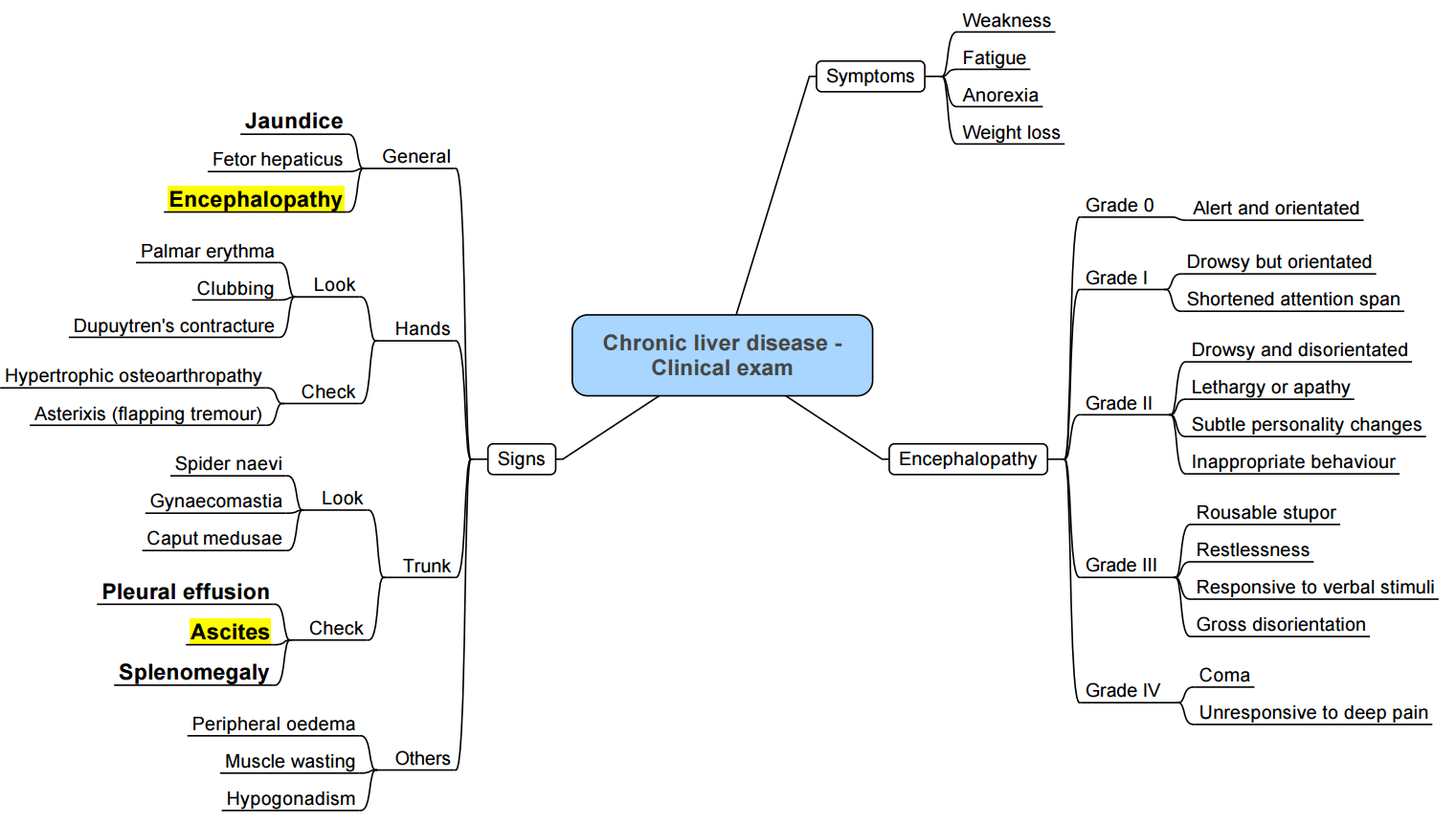
Examination
Conduct of Anaesthesia for End-Stage Liver Disease (ESLD)
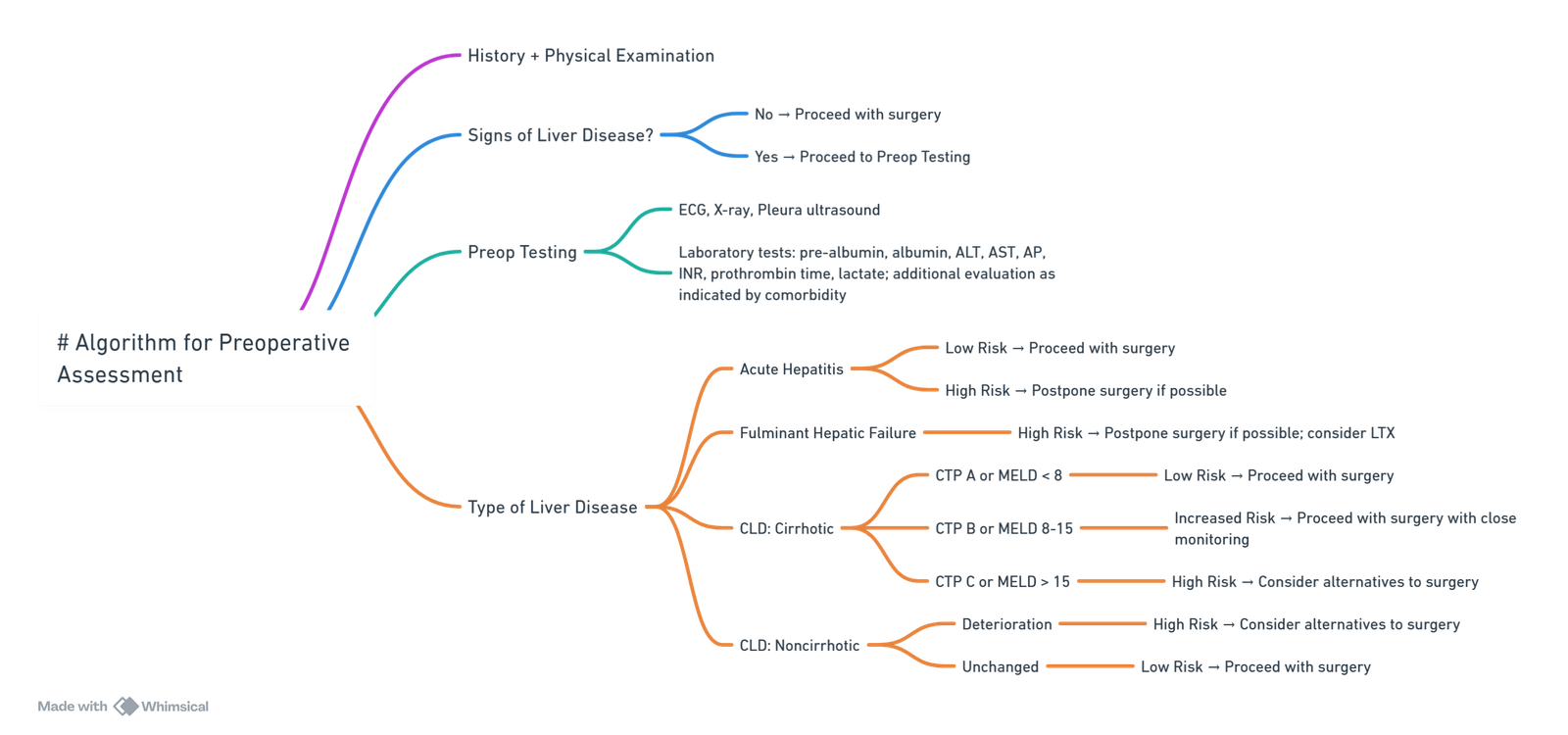
View or edit this diagram in Whimsical.
Risk Stratification
Scoring Systems
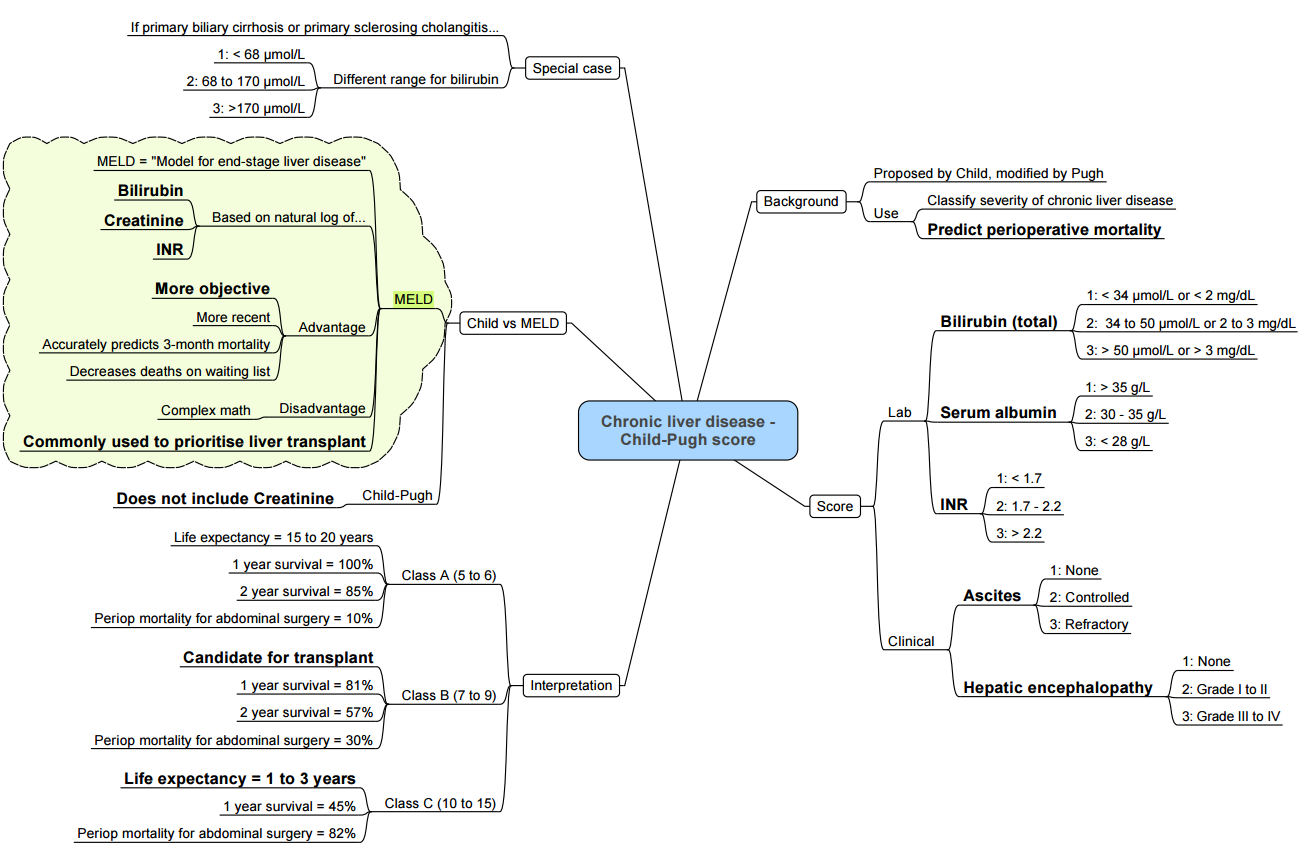
| Tool | Components | Utility | Thresholds of concern |
|---|---|---|---|
| Child–Turcotte–Pugh (CTP) | Encephalopathy, ascites, bilirubin, albumin, INR | Fast bedside estimate of synthetic failure | Class C → very high peri-operative mortality |
| MELD-Na | Creatinine, bilirubin, INR, Na⁺ | Predicts 90-day mortality better than MELD | >15–18 → defer non-urgent surgery |
| VOCAL-Penn | Age, ASA, albumin, platelet count, ascites, encephalopathy, type & urgency of surgery | Superior discrimination for 30- & 90-day mortality | Use online calculator pre-operatively |
| ASA-PS | Global status | Correlates with outcome across specialties | ASA IV–V ↔ very high risk |
Patient-Specific Predictors of Poor Outcome
- Age > 70 yr, sarcopenia, frailty
- Acute infection or sepsis
- Active alcohol use/acute alcoholic hepatitis
- Renal dysfunction (Cr > 106 µmol L⁻¹ or HRS)
- Ascites refractory to diuretics
- Upper GI bleeding within 2 weeks
- Pulmonary hypertension, cardiomyopathy, prolonged QT
Surgical Risk Categories
- Very high risk–emergency laparotomy, oesophagectomy, open cardiac or thoracic surgery
- High risk–elective intra-abdominal, major orthopaedic, open cholecystectomy
- Intermediate/low risk–endoscopy, cataract, superficial surgery
- Elective surgery should be deferred in: CTP C, MELD-Na > 18, acute decompensation or alcoholic hepatitis, severe coagulopathy (INR > 2.0) or bilirubin > 190 µmol L⁻¹.
Pharmacology in Cirrhosis
General Principles
- ↓ Albumin → ↑ free fraction of highly protein-bound drugs
- Portosystemic shunting → ↑ oral bio-availability
- Phase I (CYP450) metabolism markedly reduced; Phase II (glucuronidation) relatively preserved until late disease
- Ascites ↑ Vd of water-soluble agents
- Beware renal impairment & HRS → ↓ clearance of renally excreted drugs
Drug-Class Considerations
| Class | Recommendation in ESLD |
|---|---|
| Volatile agents | Halothane/enflurane obsolete. Use sevoflurane or desflurane (≤5 % & 0.02 % metabolism; maintain hepatic blood flow). Isoflurane acceptable. |
| IV induction | Propofol: normal induction dose, ↓ maintenance dose. Etomidate for haemodynamic instability. Avoid ketamine (sympathomimetic, ↑ ICP). |
| Opioids | Remifentanil (ester hydrolysis) ideal. Single bolus fentanyl/sufentanil safe; avoid prolonged infusions. Reduce morphine dosing (impaired glucuronidation). |
| Benzodiazepines | Prefer lorazepam/oxazepam/temazepam (Phase II). Avoid diazepam & midazolam where possible. |
| NMBAs | Cisatracurium/atracurium (Hofmann elimination) preferred. Rocuronium & vecuronium: longer duration; consider quantitative NMT & sugammadex (renal excretion, but effective). Prolonged suxamethonium effect (↓ cholinesterase)–avoid if feasible. |
| Reversal | Sugammadex 2–4 mg kg⁻¹ effective; clearance modestly prolonged but clinically acceptable. |
| Uterotonics/ergometrine | Avoid in portal hypertension (↑ afterload & BP spikes). |
Coagulation–“Rebalanced Haemostasis”
- Conventional tests (PT/INR, aPTT) reflect only pro-coagulant deficiency and do not predict bleeding.
- Use viscoelastic testing (TEG/ROTEM/Quantra) to guide transfusion.
- Target thresholds before major surgery:
- Platelets > 50 × 10⁹ L⁻¹ (higher for neurosurgery)
- Fibrinogen > 1.5 g L⁻¹ (use cryoprecipitate or fibrinogen concentrate)
- Correct factor deficiency with PCC (25–30 IU kg⁻¹) rather than large-volume FFP.
- Balance bleeding vs thrombosis–institute pharmacological VTE prophylaxis once haemostasis secure.
Pre-operative Optimisation Checklist
- Investigations–FBC, U&E, LFTs, coagulation profile, viscoelastic test, serum Na⁺, Cr, ECG, echocardiography (if dyspnoea or known cardiomyopathy), chest X-ray, ascitic tap if infection suspected.
- Ascites–large-volume paracentesis with 8 g albumin per litre removed; consider TIPS in refractory ascites.
- Nutrition & sarcopenia–pre-hab, high-protein diet ± branched-chain amino acids.
- Encephalopathy–identify precipitants; optimise lactulose (2–3 soft stools d⁻¹); add rifaximin for secondary prophylaxis.
- Infection–screen & treat SBP, UTI, pneumonia; give peri-operative antibiotics.
- Haemodynamics–cautious diuresis; avoid nephrotoxins; manage portal hypertension (non-selective β-blocker → stop 24 h pre-op if refractory ascites or hypotension).
- Drugs–stop NSAIDs; review anticoagulants/antiplatelets with hepatology/haematology input.
Intra-operative Management
Monitoring
- Standard ASA monitoring plus: invasive ABP, quantitative NMT, processed EEG (BIS), temperature, Foley catheter ± bladder pressure for large resections; consider CO monitor.
Induction & Maintenance
- Rapid-sequence induction if tense ascites/varices.
- Choose anaesthetic plan (TIVA vs volatile) based on haemodynamics & liver blood flow.
- Maintain MAP within 10 % of baseline; avoid prolonged hypotension (>100 s below MAP 65 mmHg).
Vasoactive Strategy
| Goal | First-line | Comments |
|---|---|---|
| MAP support | Norepinephrine 0.02–0.2 µg kg⁻¹ min⁻¹ | Maintains renal & splanchnic perfusion better than phenylephrine. |
| Refractory vasoplegia | Add vasopressin 0.01–0.04 U min⁻¹ | Improves renal perfusion; caution intracranial hypertension. |
| Low CO / high SVR | Dobutamine 2–5 µg kg⁻¹ min⁻¹ | Preserves splanchnic flow versus adrenaline alone. |
Fluid & Transfusion
- Use balanced crystalloids; albumin preferable to saline if large volumes
- Restrictive transfusion (Hb trigger ~75 g L⁻¹) guided by viscoelastic results.
- Avoid excessive chloride and glucose-free solutions (risk of hyponatraemia)
Regional Techniques
- Epidural/neuraxial possible if platelet count > 70 × 10⁹ L⁻¹, fibrinogen > 1.5 g L⁻¹, and normal viscoelastic clot strength; discuss with multidisciplinary team
Post-operative Care
- High Dependency or ICU admission for CTP B/C, MELD-Na > 12, major surgery, haemodynamic instability or encephalopathy.
- Vigilant monitoring for: bleeding, AKI, hypoglycaemia, sepsis, respiratory failure, arrhythmias (prolonged QT).
- Early mobilisation, adequate analgesia (regional techniques, paracetamol ≤3 g day⁻¹, avoid NSAIDs).
- Restart VTE prophylaxis (e.g., low-molecular-weight heparin) when bleeding risk acceptable.
- Daily review of electrolytes, weight, fluid balance; maintain Na⁺ 130–145 mmol L⁻¹.
Management of Common ESLD Complications
Massive Variceal Haemorrhage
- RSI with two suction devices; large-bore IV ± rapid infuser.
- Resuscitate with ratio-guided MTP; target Hb > 70 g L⁻¹, INR < 1.8, fibrinogen > 1.5 g L⁻¹, platelets > 50 × 10⁹ L⁻¹.
- Pharmacology: Octreotide 50 µg bolus then 50 µg h⁻¹, initiate ceftriaxone 1 g IV q12h.
- Endoscopic ligation once haemodynamically stable; balloon tamponade or TIPS as salvage.
Hepatic Encephalopathy & Intracranial Hypertension
- Secure airway in West Haven grade III–IV.
- Target PaCO₂ 4.5–5.0 kPa; avoid profound hyperventilation (>30 mmHg) except as rescue.
- Osmotherapy: Mannitol 0.5–1 g kg⁻¹ or hypertonic saline to Na⁺ 145–155 mmol L⁻¹.
- Maintain normothermia; treat seizures promptly (levetiracetam).
- Consider CRRT for ammonia >150 µmol L⁻¹ or refractory oedema.
- Early referral to transplant centre for ALF or ACLF grade 2–3.
Clinical Grading (West Haven)
| Grade | Level of Consciousness / Cognitive Function | Psychiatric Symptoms | Neuromuscular Function |
|---|---|---|---|
| 1 | Sleep disturbance, mild confusion | Euphoria/depression | Tremor, incoordination, asterixis |
| 2 | Moderate confusion, disorientation to time | Irritability | Asterixis, slurred speech, impaired handwriting |
| 3 | Marked confusion, somnolence | Anxiety or apathy | Asterixis, ataxia, hyperactive reflexes |
| 4 | Coma, non-command following | – | Coma, dilated pupils, loss of cranial nerve reflexes |
Summary of Key Anaesthetic Recommendations
- Stratify risk using CTP, MELD-Na and VOCAL-Penn; postpone elective procedures if modifiable risks remain.
- Optimise ascites, nutrition, infection and coagulopathy pre-operatively.
- Tailor anaesthetic drugs to reduced CYP450 activity and altered protein binding; favour agents independent of hepatic metabolism.
- Guide haemostasis with viscoelastic tests; treat both hypoand hyper-coagulability.
- Plan post-operative destination early; anticipate renal, pulmonary and neurologic complications.
Outline of Management
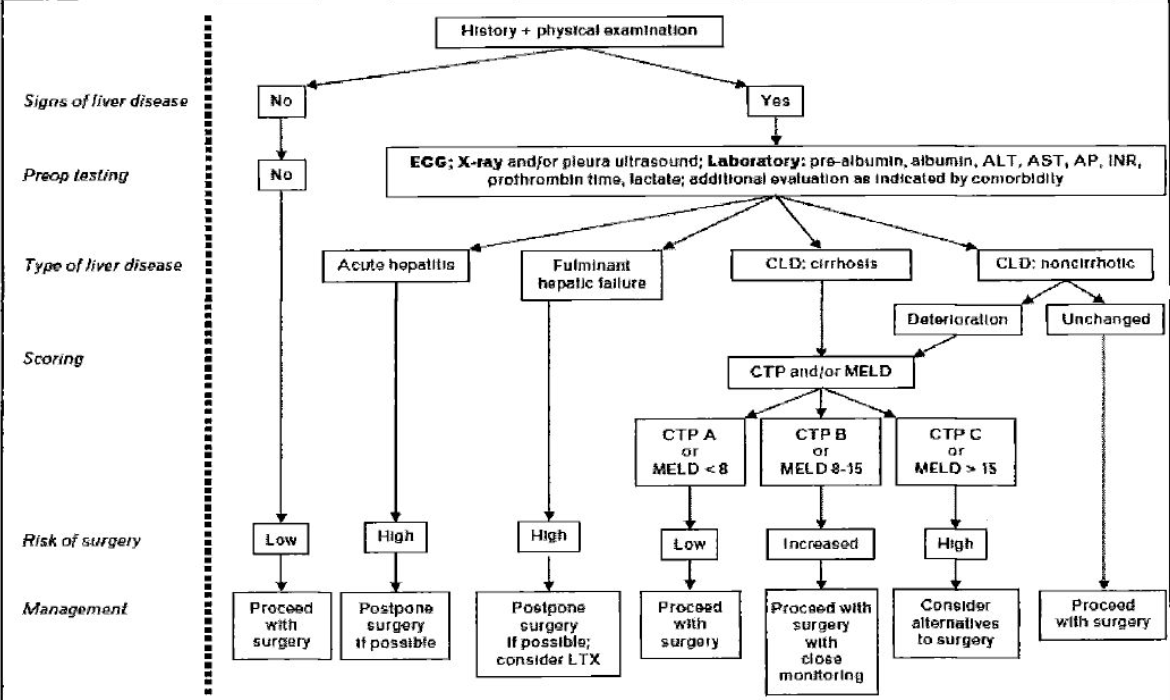
Links
References:
- Gilbert-Kawai N, Hogan B, Milan Z. Perioperative management of patients with liver disease. BJA Educ. 2022;22(3):111-117. https://pubmed.ncbi.nlm.nih.gov/35211328/ pubmed.ncbi.nlm.nih.gov
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on extra-hepatic abdominal surgery in patients with cirrhosis. J Hepatol. 2025;73:xxx-xxx. journal-of-hepatology.eu
- Mahmud N, Fricker Z, Hubbard RA, et al. Novel risk prediction models for post-operative mortality in patients with cirrhosis (VOCAL-Penn). Hepatology. 2020;72:1138-1149. med.upenn.edu
- Wong NZ, Mahmud N. Assessing the risk of surgery in patients with cirrhosis. Hepatol Commun. 2023;7(4):e38-e48. journals.lww.com
- Tripodi A, Caldwell SH. Cirrhotic coagulopathy: rebalanced haemostasis. Cleve Clin J Med. 2022;89(9):523-531. ccjm.org
- Schaefer M, Rahr H, Mallett SV. How to assess haemostasis in severe liver disease. Hematology Am Soc Hematol Educ Program. 2023;2023(1):267-276. ashpublications.org
- Kovalic AJ, Khan MA, Malaver D, et al. Thromboelastography vs standard coagulation tests in cirrhosis: systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;32(3):291-302. pubmed.ncbi.nlm.nih.gov
- Gilbert PJ, Yu A, MacAskill A, et al. Viscoelastic testing in liver disease–a scoping review. Ann Hepatol. 2024;23:100812. mdpi.com
- AGA Institute. Clinical guideline on peri-operative management of patients with cirrhosis. Gastroenterology. 2023;165:1201-1224. pmc.ncbi.nlm.nih.gov
- Egan G, Bowers J. Anesthesia in patients with chronic liver disease: an updated review. J Clin Anesth. 2024;89:110-122. pubmed.ncbi.nlm.nih.gov
Summaries
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “49ad27bf-19bf-445b-89ed-5ef9de4d71c1”



