- Anaesthesia for Peripheral Vascular Disease
- Anaesthetic Considerations
- Anaesthetic and Postoperative Management in Peripheral Vascular Surgery
- Preoperative Evaluation
- Links
- Past Exam Questions
{}
Anaesthesia for Peripheral Vascular Disease
Introduction
- Incidence: Approximately 20% in individuals over 60 years in Europe and America.
- Affected Areas: Lower limb >> cerebral > renal > mesenteric circulations.
- Cause: Predominantly atherosclerosis, a chronic systemic inflammatory disease affecting all circulations.
- Co-prevalence with CAD: High; clinical history and ECG detect only 20-40% of coexisting CAD, while cardiac catheterisation detects CAD in up to 90% of PVD patients.
- Mortality Risk: Patients have a fivefold greater risk of cardiovascular (CV) mortality and a threefold greater risk of all-cause mortality.
Management Options for Peripheral Vascular Disease (PVD)
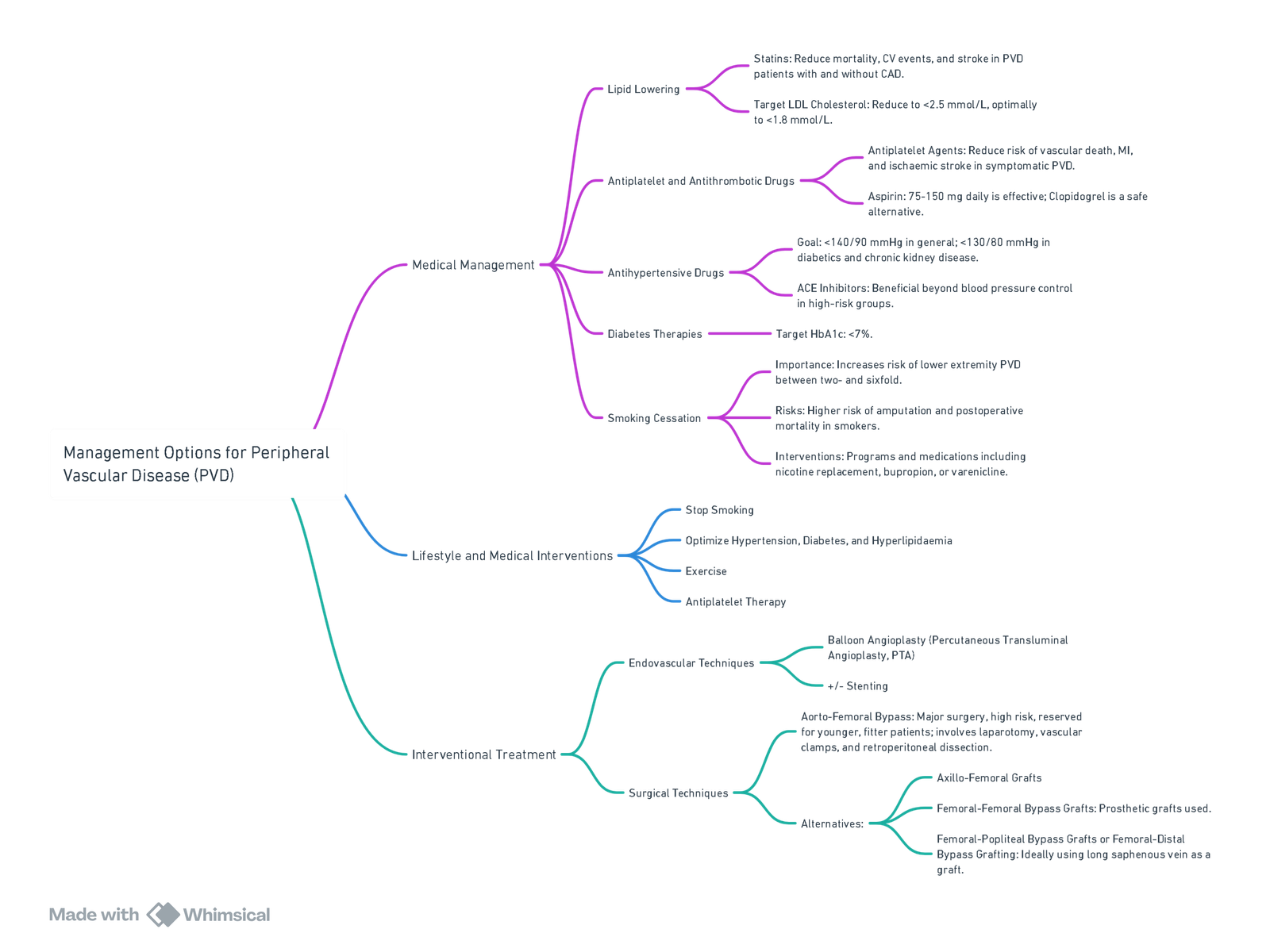
View or edit this diagram in Whimsical.
Classification and Management of Peripheral Arterial Disease (PAD)
Fontaine Classification of Peripheral Arterial Disease
| Grade | Category | Clinical Description | Objective Criteria |
|---|---|---|---|
| 0 | 0 | Asymptomatic – no hemodynamically significant occlusive disease | Normal treadmill or reactive hyperemia test |
| I | 1 | Mild claudication | Completes treadmill exercise; Ankle Pressure (AP) after exercise > 50 mmHg but at least 20 mmHg lower than resting value |
| II | 2 | Moderate claudication | Between categories 1 and 3 |
| II | 3 | Severe claudication | Cannot complete standard treadmill exercise, and AP after exercise < 50 mmHg |
| III | 4 | Ischemic rest pain | Resting AP < 40 mmHg, flat or barely pulsatile ankle or metatarsal Pulse Volume Recording (PVR); Toe Pressure (TP) < 30 mmHg |
| IV | 5 | Minor tissue loss – non-healing ulcer, focal gangrene with diffuse pedal ischemia | Resting AP < 60 mmHg, ankle or metatarsal PVR flat or barely pulsatile; TP < 40 mmHg |
| IV | 6 | Major tissue loss – extending above TM level, functional foot no longer salvageable | Same as category 5 |
Rutherford Classification System for Staging of Ischemia and Management in Patients With a Cold and Painful Leg
| Category | Clinical Features | Management |
|---|---|---|
| Category I | Normal motor function, no sensory loss, intact capillary refill | Urgent revascularization and anticoagulation |
| Category IIa | Slow to intact capillary refill, sensory loss limited to toes, no motor weakness | Emergency revascularization and anticoagulation |
| Category IIb | Slow to absent capillary refill, sensory loss involving more than toes and rest pain, motor weakness present | Emergency revascularization and anticoagulation |
| Category III | Complete loss of motor function, complete sensory loss, absent capillary refill | Primary amputation |
Risk Stratification
- Guidelines: AHA/ACC guidelines and Lee’s Revised Cardiac Risk Index (RCRI).
- Limitations of RCRI: Performs well in excluding low-risk patients but less so in predicting events in intermediate and high-risk patients, especially in vascular non-cardiac surgery.
- Emerging Biomarkers: BNP/ProBNP and Troponins for improved risk stratification.
Vascular Procedural risk
| Risk Level | Estimated MACE Risk | Examples of Procedures |
|---|---|---|
| High Risk | > 5% | – Open thoracic or abdominal aortic aneurysm repair – Infrainguinal bypass – Open renal or mesenteric artery reconstruction |
| Intermediate Risk | 1%–5% | – Endovascular aneurysm repair (EVAR) – Renal or mesenteric artery stenting – Carotid stenting or endarterectomy – Major limb amputation |
| Low Risk | < 1% | – Arteriovenous (AV) fistula creation – Superficial venous procedures – Digital amputations |
ProBNP
- Secretion: By ventricular myocytes in response to increased wall stress from volume or pressure load or ischaemia.
- Evidence: Elevated preoperative BNP/ProBNP associated with major adverse cardiac events (MACE) and higher mortality.
- Risk Prediction: Better than traditional clinical predictors; improves risk discrimination when used with RCRI.
- Thresholds: Variability in BNP/ProBNP thresholds reported in studies
- The VISION study (Vascular events In noncardiac Surgery patients cOhort evaluatioN) was a large, prospective, international study designed to evaluate major vascular complications after non-cardiac surgery. One of its key findings was the prognostic value of preoperative NT-proBNP (N-terminal pro-B-type natriuretic peptide) levels in predicting 30-day cardiovascular outcomes, especially myocardial injury after noncardiac surgery (MINS).
Preoperative NT-proBNP Risk Thresholds (VISION Study)
| NT-proBNP Level (pg/mL) | Risk Category | Associated Risk |
|---|---|---|
| < 100 | Low risk | Reference group |
| 100–199 | Moderate risk | ~2× higher risk of MINS |
| 200–1499 | High risk | ~3–4× higher risk of MINS |
| ≥ 1500 | Very high risk | ~10× higher risk of MINS, increased mortality risk |
- These thresholds were independent predictors of postoperative cardiac events, even in patients without known heart disease.
Clinical Implications
- NT-proBNP ≥ 300 pg/mL is often used as a trigger to consider cardiology referral or intensify perioperative monitoring.
- The higher the NT-proBNP, the more likely MINS, heart failure, or mortality within 30 days post-op.
- Helps guide decision-making around:
- Preoperative cardiac optimization
- Anaesthesia choice and intraoperative monitoring
- Postoperative telemetry or ICU care
Troponin T (Trop-T)
- Mechanism: Released due to myocardial necrosis, pivotal in diagnosing acute coronary syndromes (ACS).
- Non-Surgical Setting: Elevated troponins predict poor outcome and mortality.
- Surgical Setting: Elevated high-sensitive Troponin T (Tnt) post-surgery predicts 30-day mortality.
- Mortality Rates: Vary with troponin levels; even low elevations associated with increased mortality.
- Monitoring: Recommended for patients with multiple risk factors and those undergoing high-risk surgery.
- Post op monitoring 4872 hours
- Interventions: Ongoing research on effective interventions for troponin-positive patients; includes aspirin, clopidogrel, beta-blockers, and intensive surveillance.
- Interpretation (hs-cTnT Level (ng/L))
- Any post of value >65: MINS
- 20-64 any absolute change of 5: MINS
- Less than 20: Not MINS
Breakdown of Thresholds from VISION
| hs-cTnT Level (ng/L) | Interpretation in VISION |
|---|---|
| < 5 | Below detection limit (normal) |
| 5–19.9 | Mild elevation, not MINS, unless clear ischemic pattern and alternative explanation ruled out |
| ≥ 20 (with rise/fall) | MINS, if no non-ischemic cause identified |
| ≥ 65 (single value) | Considered MINS even without dynamic change |
Clinical Implications
- MINS occurs in ~8% of patients ≥45 years old after non-cardiac surgery.
- 93% of MINS cases had no ischemic symptoms.
- Associated with >10% 30-day mortality.
- Routine postoperative troponin monitoring is recommended in:
- Age ≥65 OR
- Age ≥45 with significant CV disease
- Recommended days: Postoperative Day 0, 1, 2, and optionally 3
South African Cardiovascular Risk Management in Elective Non-Cardiac Surgery (2021)
Patient Selection Criteria
Indications for Risk Stratification and Monitoring:
- Patients ≥45 years undergoing elective non-cardiac surgery with a history of:
- Coronary artery disease.
- Congestive cardiac failure.
- Stroke or transient ischaemic attack (TIA).
- Patients ≥18 years undergoing vascular surgery with:
- Peripheral vascular disease.
Justification: These populations have an estimated 30-day major adverse cardiac event (MACE) risk >5%.
- Peripheral vascular disease.
Risk Stratification Recommendations
1. Routine Non-Invasive Testing
- Recommendation: Not recommended for cardiovascular risk stratification in adults prior to elective non-cardiac surgery.
- Strength: Strong
- Evidence Quality: Low-to-moderate
2. Natriuretic Peptide (NP) Screening
- Recommendation: NP testing (BNP or NT-proBNP) is recommended for all patients meeting the above selection criteria.
- Thresholds for abnormal values:
- BNP > 99 pg/mL
- NT-proBNP > 300 pg/mL
- Strength: Strong
- Evidence Quality: High
3. Postoperative Troponin Monitoring
- Recommendation: Daily troponin measurement for 48–72 hours postoperatively in selected patients:
- If no preoperative NP testing was done.
- Or if preoperative NP is elevated (above thresholds).
- Strength: Conditional
- Evidence Quality: Moderate
Perioperative Risk Assessment and Monitoring Algorithm
- Target Population:
- Same as above patient selection criteria.
- Determine NP Testing Availability:
- If available:
- Perform BNP or NT-proBNP testing.
- Abnormal result (BNP > 99 pg/mL or NT-proBNP > 300 pg/mL):
- Proceed with postoperative troponin monitoring.
- Normal result:
- No further cardiac intervention required.
- If not available:
- Proceed directly to postoperative troponin monitoring.
- If available:
What if the ProBNP is >300 pre Op
- Elevated NT-proBNP (>300 pg/mL) Before Non-Cardiac Surgery:
- Indicates high risk for post-op cardiac events.
- No need for routine further testing if asymptomatic and functional status is good.
- Consider echo or stress testing only if:
- Symptoms/signs of cardiac disease, or
- Poor functional capacity + high-risk surgery.
- Monitor troponin post-op (Days 1–3)
- Focus on optimizing perioperative care, not delaying surgery.
Anaesthetic Considerations
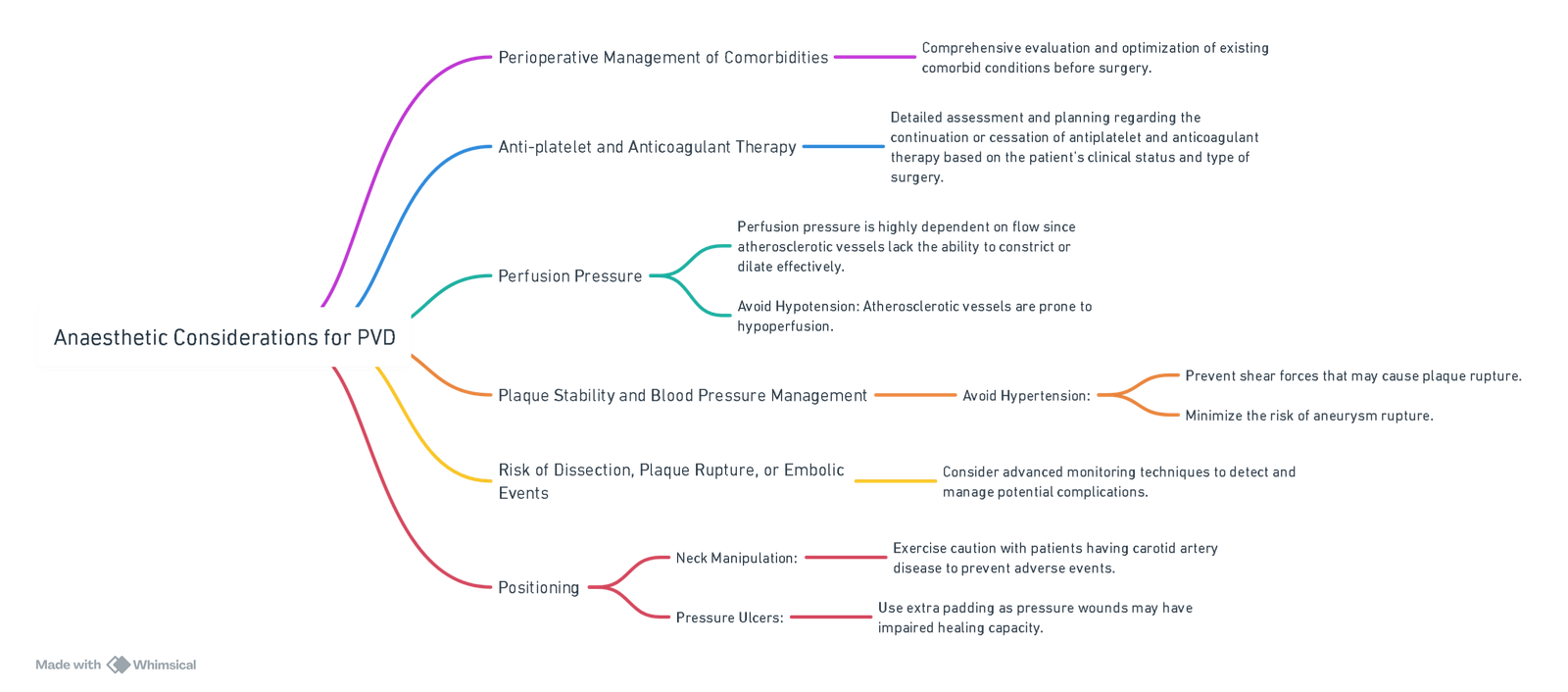
View or edit this diagram in Whimsical.
Anaesthetic and Postoperative Management in Peripheral Vascular Surgery
| Operation | Anesthetic | Monitoring | Postoperative Analgesia |
|---|---|---|---|
| Aortobifemoral bypass graft | General Anesthesia (GA) (IPPV via ETT) plus thoracic epidural | Routine plus A-line and CV-line; consider CO monitor | Epidural for 2–3 days, then PCA opioid, then oral analgesia |
| Axillofemoral bypass graft | GA (IPPV via ETT) | Routine | PCA or oral opioid |
| Femoro-femoral / Femoro-popliteal / Femoro-distal bypass grafts / Femoral endarterectomy | Regional (lumbar epidural; CSE; spinal) plus sedation or GA (IPPV via ETT, or spontaneous ventilation via LMA) | Routine (A-line and/or CV-line not usually required unless specific patient features present) | Oral opioid (consider epidural infusion of LA for selected patients) |
| Balloon embolectomy | LA infiltration by surgeon plus intravenous sedation/analgesia from anesthetist | Routine | Simple oral analgesia ± oral opioid |
Preoperative Evaluation
Risk Stratify
- As above
- Stick to a single guideline.
- ProBNP and Trop T for qualifying candidates
Optimization
| Risk Factor | Target |
|---|---|
| Glycemic control | HbA1C < 7% |
| LDL-cholesterol | < 1.8 mmol/L |
| Smoking | Complete nicotine cessation |
| Arterial pressure | < 130/80 mmHg |
| Anti-platelets | Aspirin or clopidogrel |
Pharmacotherapy Prior to Major Vascular Surgery
Beta-Blockers
- Should not be started de novo in low-risk patients.
- If chronically on beta-blocker therapy, continue if no contraindications.
- If multiple RCRI risk factors, high-risk myocardial ischemia, or heart failure, can consider starting a beta-blocker. If started, should be started at least 7 days prior to operative intervention.
ACEI/ARB
- Should not be started de novo.
- If chronically on ACEI/ARB therapy, continue if no contraindications. If discontinued, should be restarted soon in the postoperative setting when feasible.
- If heart failure with reduced ejection fraction, reasonable to start prior to operative intervention. If started, should be started at least 7 days prior.
Statins
- Continue on home statin or start on statin prior to operative intervention.
Antiplatelets
- If no prior PCI, there is no benefit to starting aspirin prior to major vascular, noncardiac surgery.
- Start low-dose aspirin prior to CEA and continue in the postoperative period.
- If prior PCI and patient is on single antiplatelet therapy chronically, continue single antiplatelet therapy.
- If prior PCI and within 1 month of BMS or 6 months of DES, should continue DAPT.
Anticoagulants
- VKA should be held 3–5 days prior to major vascular surgery, for an INR ≤ 1.5 prior to operative intervention.
- Apixaban, rivaroxaban, and dabigatran (with CrCl ≥ 50 mL/min) should be withheld 2 days prior to intervention and resumed 2–3 days following intervention. Dabigatran (with CrCl < 50 mL/min) should be withheld for 4 days prior to intervention.
- No bridging is required for atrial fibrillation alone.
- For mechanical heart valves, if a current generation mechanical aortic valve (e.g., On-X bileaflet valve) alone without thromboembolic risk factors (i.e., atrial fibrillation, previous thromboembolism, hypercoagulable state, left ventricular systolic dysfunction), bridging anticoagulation may be deferred. All other mechanical heart valves or if thromboembolic risk factors should be bridged with low-molecular-weight or unfractionated heparin.
Novel Diabetic Agents
- GLP-1 analogues and SGLT2 inhibitors should be withheld prior to operative intervention.
Summary of the ACC/AHA, ESC/ESA, and CCS Recommendations on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery
Electrocardiogram
- ACC/AHA: If undergoing intermediate to high-risk surgery and with coronary artery disease, arrhythmia, peripheral arterial disease, cerebrovascular disease, or structural heart disease.
- ESC/ESA: If undergoing high-risk surgery.
- CCS: No recommendation.
Resting Echocardiogram
- ACC/AHA: Obtain if undergoing intermediate to high-risk surgery and with decompensated heart failure, valvular disease, structural heart disease, or dyspnea of unclear etiology.
- ESC/ESA: May be considered if undergoing high-risk surgery.
- CCS: Do not obtain unless severe valvular disease, pulmonary hypertension, or an undiagnosed cardiomyopathy is present.
Stress Testing
- ACC/AHA:
- Low risk of MACE (< 1%): Do not obtain.
- Elevated risk of MACE (≥ 1%) based on METs:
- < 4 METs: Obtain.
- 4-10 METs: Reasonable to forgo.
- More than 10 METs: Forgo.
- ESC/ESA:
- High-risk surgery:
- < 4 METs and have at least one clinical risk factor (ischemic heart disease, heart failure, stroke/transient ischemic attack, renal dysfunction, or insulin-dependent diabetes).
- High-risk surgery:
- CCS: Do not obtain.
Coronary Angiography
- ACC/AHA:
- Routine coronary angiography not recommended.
- Coronary angiography and revascularization indicated for acute coronary syndrome.
- ESC/ESA: Same as ACC/AHA.
- CCS: Coronary angiography and revascularization indicated for acute coronary syndrome.
Links
- Vascular surgery
- Vascular physiology
- Endovascular Abdominal Aortic Aneurysm Repair (EVAR)
- Thoracic pre-op assessment
- Cardiac for non-cardiac surgery
Past Exam Questions
Preoperative Assessment for Vascular Surgery
A 72-year-old obese male smoker with hypertension presents for elective femoral-popliteal arterial bypass surgery.
Discuss the goals of pre-operative assessment, risk stratification, and how you would structure your report. (10)
Aorto-Bifemoral Bypass Preoperative Assessment
a) What is his risk for developing an adverse event post-operatively and why? (2)
b) How should the following drugs be managed pre-operatively?
- i) Dabigatran. (2)
- ii) Digoxin. (2)
- iii) Diltiazem. (2)
c) Would you start this patient on a beta-blocker preoperatively? Motivate your answer. (2)
References:
- Neves SE. Anesthesia for Patients with Peripheral Vascular Disease and Cardiac Dysfunction. Anesthesiol Clin. 2016 Dec;34(4):775-795. doi: 10.1016/j.anclin.2016.06.011. PMID: 27816134.
- Tovey, G. and Thompson, J. P. (2005). Anaesthesia for lower limb revascularization. Continuing Education in Anaesthesia Critical Care &Amp; Pain, 5(3), 89-92. https://doi.org/10.1093/bjaceaccp/mki024
- Alphonsus CS, Naidoo N, Motshabi Chakane P, Cassimjee I, Firfiray L, Louwrens H, Van der Westhuizen J, Malan A, Spijkerman S, Kluyts H, Cloete NJ, Kisten T, Nejthardt NB, Biccard BM. South African cardiovascular risk stratification guideline for non-cardiac surgery. S Afr Med J. 2021 Oct 29;111(10b):13424. PMID: 34949237.
- Abbott TEF, Pearse RM, Archbold RA, Ahmad T, Niebrzegowska E, Wragg A, Rodseth RN, Devereaux PJ, Ackland GL. A Prospective International Multicentre Cohort Study of Intraoperative Heart Rate and Systolic Blood Pressure and Myocardial Injury After Noncardiac Surgery: Results of the VISION Study. Anesth Analg. 2018 Jun;126(6):1936-1945. doi: 10.1213/And.0000000000002560. PMID: 29077608; PMCID: PMC5815500.
- Lee C, Columbo JA, Stone DH, Creager MA, Henkin S. Preoperative evaluation and perioperative management of patients undergoing major vascular surgery. Vasc Med. 2022 Oct;27(5):496-512. doi: 10.1177/1358863X221122552. PMID: 36214163; PMCID: PMC9551317.
- FCA Part II Anaesthetic Refresher Course 2013: University of the Witwatersrand PVD: Dr I Shaikh
Summaries:
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “0352632f-5032-47e2-a9ee-c484a4e51e21”



