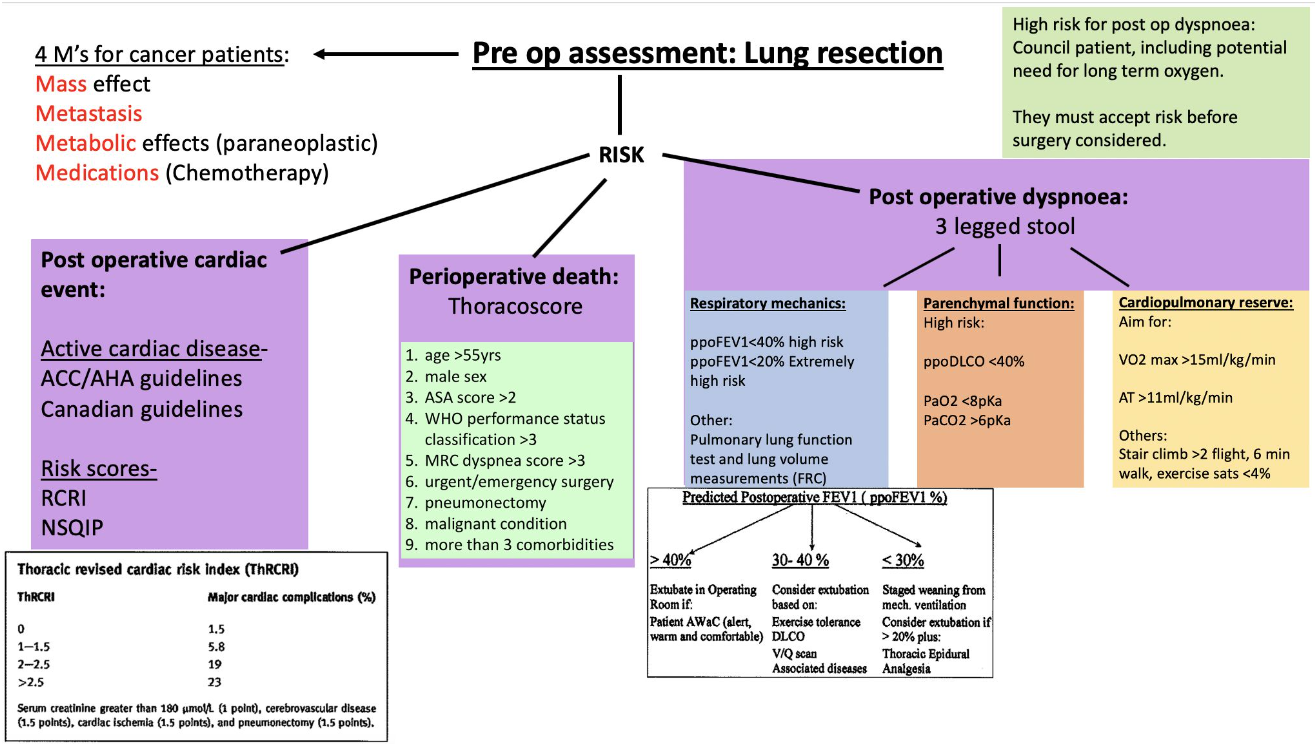
- Pre-operative Risk Assessment for Thoracic (Lung Resection) Surgery
- Detailed Explanation of 3-Legged Approach
- Cardiopulmonary Interaction
{}
Pre-operative Risk Assessment for Thoracic (Lung Resection) Surgery
- Key principle: No single test is sufficient. Combine lung mechanics, gas-exchange capacity and cardiopulmonary reserve (“three-legged stool”) to predict whether the remaining lung will support extubation and recovery.
Introduction
- All patients undergoing pulmonary resections should have a preoperative assessment of their respiratory function in three areas:
- Lung mechanical function
- Pulmonary parenchymal function
- Cardiopulmonary reserve (the “three-legged stool” of respiratory assessment)
- Following pulmonary resection surgery, patients with adequate predicted postoperative respiratory function can often be weaned and extubated in the operating room if they are “AWaC” (alert, warm, and comfortable)
Summary of Initial Assessment
Structured Screening Pathway
| Step | All patients | “Screen-positive” criteria | Next investigation |
|---|---|---|---|
| 1. Basic assessment | History, comorbidity review (4 M’s in malignancy: Mass, Metabolic, Metastases, Medications); exercise tolerance; baseline ABG; chest CT | Inability to climb > 2 flights or walk > 400 m; FEV₁ < 80 % predicted; dyspnoea MRC ≥ 3 | Proceed to Step 2 |
| 2. Spirometry | Calculate ppoFEV₁ ppoFEV₁ = pre-op FEV₁ × (1–segments removed ÷ 19) |
ppoFEV₁ < 40 % or < 1.5 L (lobectomy) / < 2 L (pneumonectomy) | Full PFT inc. DLCO |
| 3. Gas exchange | Measure DLCO; derive ppoDLCO | ppoDLCO < 40 % or hypoxia (PaO₂ < 8 kPa) / hypercapnia (PaCO₂ > 6 kPa) | CPET or low-tech exercise tests |
| 4. Cardiopulmonary reserve | Formal CPET (VO₂max) if available | VO₂max < 15 mL kg⁻¹ min⁻¹–high risk | Consider sub-max tests: 6-min walk, stair climb, shuttle walk |
| 5. Regional function | V/Q or quantitative CT if pneumonectomy planned & either ppoFEV₁ or ppoDLCO < 40 % | — | Refine ppo values and operative plan |
- Implement smoking cessation, bronchodilation and pulmonary rehab in parallel with testing.
Evidence-Based Thresholds
| Parameter | Acceptable | Increased risk | Contra-indication / consider non-surgical therapy |
|---|---|---|---|
| ppoFEV₁ | > 40 % predicted | 30–40 % → base decision on exercise tests & DLCO | < 30 % (or < 0.85 L) |
| ppoDLCO | > 40 % | 30–40 % | < 30 % |
| VO₂max | ≥ 20 mL kg⁻¹ min⁻¹ | 10–20 mL kg⁻¹ min⁻¹ | < 10 mL kg⁻¹ min⁻¹ |
| 6-min walk | ≥ 500 m | 250–500 m | < 250 m or SpO₂ fall > 4 % |
| Stair climb | ≥ 5 flights (≈ 20 m) | 2–4 flights | < 2 flights |
Anaesthetic Implications
| Predicted function | Extubation plan | Post-op destination |
|---|---|---|
| ppoFEV₁ & ppoDLCO ≥ 40 % and VO₂max ≥ 15 | Awake extubation in theatre if AWaC | HDU |
| Borderline values | Trial extubation if regional analgesia optimal, low FiO₂, PaCO₂ < 6 kPa | HDU/ICU step-down |
| ppoFEV₁ or ppoDLCO < 30 % or VO₂max < 10 | Planned overnight ventilation, staged wean | ICU |
Detailed Explanation of 3-Legged Approach
Respiratory Function Assessment
- Major respiratory complications such as atelectasis, pneumonia, and respiratory failure occur in 15–20% of patients and account for the majority of the expected 3–4% mortality
- Cardiac complications such as arrhythmia and ischemia occur in 10–15% of the thoracic population
- Patients at increased risk of respiratory complications (ppoFEV1 <40%) should undergo complete pulmonary function testing, including assessment of lung volumes and airway resistance. Two basic methods of measurement are insoluble gas dilution and plethysmography.
Spirometry
- All patients undergoing pulmonary resection should have preoperative spirometry to assess the forced expiratory volume in 1 second (FEV₁).
- Results provide baseline forced expiratory volume in 1 second (FEV1), measured as a percentage of predicted normal (FEV1%).
- Predicted postoperative FEV1 (ppoFEV₁) estimates pulmonary function after resection and guides surgical risk assessment.
Calculation of Predicted Postoperative FEV₁ (ppoFEV₁)
Segment Counting Method (19 lung segments total):
ppoFEV1 = preoperative FEV1 × {(19-number of segments removed) ÷ 19}
In practice, the total number of lung segments is usually taken as 19 (10 in the right lung and 9 in the left)
Thus, each segment represents roughly 5% of lung function. For example, if a lobectomy removes 5 segments out of 19, the predicted remaining FEV1 would be ~74% of the preoperative value.
Perfusion Method:
ppoFEV1 = preoperative FEV1 × (1–fraction of total perfusion in lung tissue to be resected)
Risk Thresholds
- ppoFEV₁ <40% indicates increased surgical risk, though a threshold of <45% may be more appropriate in elderly patients.
- ppoFEV₁ <0.85 L significantly raises risk of postoperative respiratory failure.
Preoperative and Postoperative Assessment
Assessment Criteria:
- ppoFEV1 > 80% or > 2L:
- Pneumonectomy: No further testing required
- Lobectomy: No further testing required
- ppoFEV1 < 80% or < 2L for Pneumonectomy or < 1.5L for Lobectomy:
- Perform DLCO and express as % of predicted DLCO
- Measure saturations (SpO2) on air
Risk Stratification:
- ppoFEV1 < 40% and DLCO < 40%: High risk
- ppoFEV1 > 40% and DLCO > 40% and SpO2 > 90%: Average risk (no further testing)
- Other combinations require further exercise testing
Shuttle Walk Test:
- < 25 shuttles or desaturation > 4%: High risk
- More than 25 shuttles and < 4% desaturation:
- VO2 max < 15 mL/kg/min: High risk
- VO2 max > 15 mL/kg/min: Average risk
Plethysmography
Body Plethysmography Method for Determination of FRC:
- Mouth pressure (Pm) reflects change in alveolar pressure
- Box pressure (Pm) reflects change in lung volume
- Patient seated in an airtight box, performing panting efforts against a closed shutter
Validity of Tests (ATS/ERS Guidelines)
Technicalities:
- Operator: Trained, meticulous technique
- Patient: Cooperative, sitting or standing, maximum effort
- Apparatus: Calibrated daily, appropriate fitting
- Interpretation: Correct reference values considering age, height, gender, environment
Acceptability and Reproducibility:
- Good start: No cough, hesitation, obstruction, artifacts, early termination, air leaks, or obstructed mouthpiece
- Exhalation: Complete or at least 6 seconds
- Maximum of 8 attempts for 3 reproducible efforts: At least 2 within 150 mL or 5%
Pulmonary Parenchymal Testing
- Traditionally, arterial blood gas data such as PaO2 <60 mmHg or PaCO2 >45 mmHg have been used as cut-off values for pulmonary resection.
- The most useful test of the gas exchange capacity of the lung is the diffusing capacity for carbon monoxide (DLCO).
- DLCO reflects the total functioning surface area of the alveolar-capillary interface.
- This test, included with spirometry and plethysmography by most pulmonary function laboratories, is a useful predictor of perioperative morbidity and mortality.
- The corrected DLCO can be used to calculate a post-resection (ppo) value using the same calculation as for the FEV1.
- A ppoDLCO <40% predicted correlates with increased respiratory and cardiac complications and is largely independent of the FEV1.
- The National Emphysema Treatment Trial showed that patients with a preoperative FEV1 or DLCO <20% had an unacceptably high perioperative mortality rate.
- These values are considered the minimal values compatible with a successful outcome.
Diffusing Capacity of Carbon Monoxide (DLCO)
Decreased DLCO:
- Decreased membrane surface area (emphysema)
- Increased membrane thickness (ILD)
- Anaemia
- Pulmonary hypertension
Increased DLCO:
- Pulmonary haemorrhage
- Asthma
- Polycythaemia
- Exercise
Regional Lung Function
- Prediction of post-resection pulmonary function can be refined by assessing the preoperative contribution of the lung or lobe to be resected using imaging of regional lung function, particularly useful for pneumonectomy patients.
- Regional lung function imaging should be ordered for any potential pneumonectomy patient with preoperative FEV1 and/or DLCO <80% (i.e., ppo values <40% predicted).
- Regional lung function imaging can be performed by:
- Radionuclide ventilation/perfusion (V/Q) lung scanning
- Pulmonary quantitative CT scanning
- Three-dimensional dynamic perfusion MRI
V/Q Scanning
- Ventilation/perfusion lung scanning is the gold standard.
- Regional ventilation is assessed by scanning after inhalation of a radio-labelled insoluble gas (commonly xenon-133).
- Regional lung perfusion is assessed by scanning after intravenous injection of radiolabelled particles trapped in the pulmonary capillaries (commonly technetium-99 m macroaggregated albumin).
- Postoperative lung function shows high correlation with predicted values based on preoperative V/Q scanning for FEV1 (r = 0.92), DLCO (r = 0.90), and VO2 max (r = 0.85).
- Prediction is more accurate for post-pneumonectomy versus post-lobectomy values. If there is a discrepancy between ventilation and perfusion scan results, it is preferable to use the result attributing the larger proportion of ventilation or perfusion to the diseased lung for post-resection pulmonary function estimation.
Cardiopulmonary Interaction
- After pulmonary resection, there is a degree of right ventricular dysfunction proportional to the amount of functioning pulmonary vascular bed removed. The exact aetiology and duration of this dysfunction remain unknown. Clinical evidence of this hemodynamic problem is minimal at rest but dramatic during exercise, leading to elevated pulmonary vascular pressures, limitation of cardiac output, and absence of the normal decrease in pulmonary vascular resistance with exertion.
VO2 Max
- Formal laboratory exercise testing is the “gold standard” for assessing cardiopulmonary function, with maximal oxygen consumption (VO2 max) being the most useful predictor of post-thoracotomy outcome.
- The test is performed on a bicycle ergometer or treadmill. Resting measurements are made for 3–5 minutes. Three minutes of unloaded cycling is performed as a warm-up period. The workload is incremented to reach maximum work capacity in 8–12 minutes.
- The test continues to symptom limitation (e.g., severe dyspnoea) or discontinuation by medical staff (e.g., significant ECG abnormalities) or achievement of maximum predicted heart rate.
- Estimated VO2 max is based on age, sex, and height. For sedentary males, VO2 max (mL/min) = (height (cm) − age (years)) × 20. For sedentary females, VO2 max = ((height (cm) − age (years)) × 14) / weight (kg).
- The risk of morbidity and mortality is unacceptably high if preoperative VO2 max is <15 mL/kg/min. Few patients with VO2 max >20 mL/kg/min have respiratory complications.
Anaerobic Threshold
- The anaerobic threshold, measured during exercise testing, is a predictor of postoperative complications.
- The anaerobic threshold is the exercise level at which lactate begins to accumulate in the blood and anaerobic metabolism begins, approximately 55% of VO2 max in untrained individuals but >80% in trained athletes.
- It can be documented by repeated blood lactate analysis during exercise or by a threshold increase in CO2 production above the initial respiratory quotient.
- A threshold value for the anaerobic threshold of <11 mL/kg/min suggests increased risk, but this is not well validated.
6-Minute Walk Test
- The distance a patient can walk during a 6-minute test (6MWT) correlates well with VO2 max and requires little or no laboratory equipment.
- For patients with moderate or severe COPD, the 6MWT distance can be used to estimate VO2 max by dividing by 30 (e.g., 600 m distance is equivalent to a VO2 max of 20 mL/kg/min).
Fall in SpO2
- Some centres assess the fall in oximetry (SpO2) during exercise. A decrease of SpO2 >4% during exercise indicates increased risk of morbidity and mortality.
Amount of Functional Lung Tissue Removed
- Post-resection exercise capacity can be estimated based on the amount of functioning lung tissue removed. A ppoVO2 max <10 mL/kg/min is considered a contraindication to pulmonary resection.
- Mortality was 100% (3/3) in patients with a ppoVO2 max <10 mL/kg/min in a small series.
Stair Climbing
- The ability to climb stairs is a useful test in ambulatory patients. The ability to climb five flights correlates with VO2 max >20 mL/kg/min; climbing two flights corresponds to a VO2 max of 12 mL/kg/min. Patients unable to climb two flights are at extremely high risk.
Associated Medical Conditions — Optimisation & Risk Modification
Optimise Before Theatre
| Condition | Required optimisation targets |
|---|---|
| IHD | HR < 80, MAP within 10 % baseline, β-blocker & statin on DOS |
| AF risk | Mg²⁺ > 0.8 mmol L⁻¹, K⁺ 4–4.5 mmol L⁻¹, epidural in situ |
| CKD | eGFR trend stable, no nephrotoxins, serum Cr within 20 % baseline |
| COPD | FEV₁ maximised, no active infection, able to clear secretions |
| Smoking | ≥ 4 wk abstinence ideal; absolute minimum 12 h |
| Paraneoplastic | Correct Ca²⁺ < 2.8 mmol L⁻¹; Na⁺ ≥ 130 mmol L⁻¹ |
Cardiac Disease
Ischaemic Heart Disease
- Incidence: Post-thoracotomy myocardial injury ≈ 5 %; peak on Days 2–3.
- Evaluation
- Low/intermediate clinical risk + ≥ 4 METs functional capacity → no further testing.
- Poor functional capacity (<4 METs) or unexplained dyspnoea → non-invasive stress imaging (stress echo or perfusion scan).
- Peri-operative strategy
- Continue β-blocker, statin and ACE-I.
- Avoid excessive intra-operative hypotension (MAP within 10 % baseline).
- Maintain HR < 80 min⁻¹ to limit demand.
Arrhythmias
| Factor | Comment | Mitigation |
|---|---|---|
| Incidence | AF/flutter 20–40 % (lobectomy) – 60 % (pneumonectomy) within 72 h | Telemetry 72 h; Mg²⁺ 30 mg kg⁻¹ IV if low |
| Risks | Age > 70, pneumonectomy, intra-pericardial dissection, > 500 mL blood loss | Thoracic epidural / paravertebral block; judicious fluids |
| Prophylaxis | Per 2023 ERS/ESTS guideline: Amiodarone 400 mg po × 5 d pre-op or 300 mg IV loading post-op in high-risk cases | Early mobilisation; correct electrolytes |
Geriatric Considerations
- No absolute age limit; octogenarian mortality ~3 %.
- Transthoracic echo for ≥ 70 y with COPD, murmur, heart failure or abnormal ECG.
- Frailty score ≥ 5 predicts doubled pulmonary-cardiac complication rate; initiate pre-habilitation.
Renal Dysfunction
- Risk factors: eGFR < 45 mL min⁻¹ 1.73 m⁻², cisplatin therapy, pneumonectomy, peri-operative transfusion, severe infection.
- Incidence: Post-op AKI (KDIGO) 8–12 %; triples ICU stay and 90-day mortality.
- Optimisation:
- Withhold nephrotoxic drugs, optimise volume, use buffered crystalloids.
- Target urine > 0.5 mL kg⁻¹ h⁻¹; avoid hydroxyethyl starch.
- Dose-adjust LMWH, opioids.
Chronic Obstructive Pulmonary Disease
Pre-operative Optimisation
| Intervention | Evidence | Timing |
|---|---|---|
| Bronchodilators: LABA ± LAMA, +/- inhaled steroid | ↓ post-op pneumonia (2022 GOLD meta-analysis) | Continue until DOS |
| Pulmonary rehabilitation | ↑ 6-MWD by 40 m; ↓ complications | ≥ 4 weeks; fast-track 2-week high-intensity viable |
| Physiotherapy | IS + coached deep breathing, PEP device | Start once listed |
| Treat exacerbation | ABX, steroids (pred 30 mg × 5 d) | Complete course before surgery |
Smoking Cessation
| Time before surgery | Physiological benefit |
|---|---|
| > 8 weeks | 4-fold reduction pulmonary complications |
| 4 weeks | Improved mucociliary clearance, wound healing |
| 12 h | ↓ carboxyhaemoglobin, ↑ exercise capacity 10–20 % |
- No evidence of “rebound” complications even if cessation < 8 weeks; counsel every smoker.
Malignancy-Specific Issues—“4 M’s”
| Category | Examples | Anaesthetic relevance |
|---|---|---|
| Mass | SVC syndrome, tracheal compression, Pancoast, RLN palsy | Plan awake fibre-optic or ECMO standby |
| Metabolic | HyperCa²⁺, SIADH, Cushing, Lambert-Eaton | Correct electrolytes; prepare for difficult reversal in LE myopathy |
| Metastasis | Brain, bone, liver, adrenal | Imaging if neuro Sx; avoid nitrous with brain mets |
| Medication | Bleomycin (O₂ toxicity), doxorubicin (cardiomyopathy), cisplatin (renal) | Limit FiO₂ < 0.3 after lung re-expansion; baseline echo; renal-protective fluids |
Anaesthesia Considerations for Different Types of Lung Cancer
| Type | Considerations |
|---|---|
| Squamous cell | Central lesions (predominantly), mass effects: obstruction, cavitation, hypercalcemia, hypertrophic pulmonary osteoarthropathy. |
| Adenocarcinoma | Peripheral lesions, metastases (distant), growth hormone, corticotropin. |
| Small cell | Central lesions (predominantly), few surgically treatable, paraneoplastic syndromes, Lambert–Eaton syndrome, fast growth rate, early metastases. |
| Carcinoid | Proximal, intra-bronchial, no association with smoking, 5-year survival >90%, carcinoid syndrome (rarely). |
| Mesothelioma | Intraoperative hemorrhage, direct extension to diaphragm, pericardium, etc. |
Risk Scores
Revised Cardiac Risk Index (RCRI)
| Risk Factor | Points |
|---|---|
| Cerebrovascular disease | 1 |
| Congestive heart failure | 1 |
| Creatinine level > 2.0 mg/dL | 1 |
| Diabetes mellitus requiring insulin | 1 |
| Ischemic cardiac disease | 1 |
| Supra-inguinal vascular surgery, intrathoracic surgery, or intra-abdominal surgery | 1 |
Risk of Major Cardiac Event:
| Points | Percentage risk (95% CI) |
|---|---|
| 0 | 0.4 (0.05-1.5)% |
| 1 | 0.9 (0.3-2.1)% |
| 2 | 6.6 (3.9-10.3)% |
| ≥ 3 | ≥ 11 (5.8-18.4)% |
Thoracic Revised Cardiac Risk Index
- Used for predicting major cardiac events in patients without active cardiac conditions undergoing thoracic surgery
| Risk Factor | Points |
|---|---|
| History of coronary artery disease | 1.5 |
| History of cerebrovascular disease | 1.5 |
| Pneumonectomy | 1.5 |
| Serum creatinine > 177 µmol/L | 1 |
| Points | Risk % |
|---|---|
| 0 | 0.9% |
| 1-1.5 | 4.2% |
| 2-2.5 | 8% |
| >2.5 | 18% |
Thoracoscore
- Used as a global risk score predicting mortality from thoracic surgery
| Variable | Risk Factor |
|---|---|
| Age | >55 years |
| Gender | Male |
| ASA physical status | >2 |
| Performance Status Classification (WHO) | >3 |
| Dyspnea score (Medical Research Council) | >3 |
| Surgical priority | Urgent or emergency |
| Procedure class | Pneumonectomy |
| Diagnosis | Malignant |
| Comorbid diseases | >3 |
Modified Medical Research Council (mMRC) Dyspnea Scale
| mMRC Grade | Description | Severity Grouping |
|---|---|---|
| 1 | Breathless with strenuous exercise | Mild |
| 2 | Short of breath when hurrying on the level or walking up a slight hill | Moderate |
| 3 | Walks slower than people of the same age on the level or stops for breath while walking at own pace on the level | Severe |
| 4 | Stops for breath after walking 100 m | Severe |
| 5 | Too breathless to leave the house or breathless when dressing | Severe |
METS
Energy Consumption in Metabolic Equivalents (METS)
| Activity | METS |
|---|---|
| Sitting quietly | 1 |
| Walking 1 block | 2 |
| Playing the accordion | 2 |
| Climbing 1 flight stairs | 4 |
| Sexual intercourse* | 6 |
| Bowling* | 6 |
| Ice Hockey | 8 |
| Running 6 mph | 10 |
| Cross-country ski racing | 14 |
MET = basal oxygen consumption = 3.5 mL/kg/min
Postoperative Dyspnoea
Risk Assessment for Postoperative Dyspnoea
Spirometry and Transfer Factor
- Low Risk: ppo FEV1 ≥40% and ppo TLCO ≥40%
- Moderate to High Risk: ppo FEV1 <40% and/or ppo TLCO <40%
Functional Assessment
- Good: Moderate Risk: Inform patients of risk of mild-moderate postoperative shortness of breath with surgery or radiotherapy
- Moderate/Poor: High Risk: Inform patients of risk of severe postoperative dyspnoea and/or long-term oxygen therapy with surgery or radiotherapy
Notes:
- Consider V/Q scanning for more accurate prediction of postoperative values
- High risk of ventilator dependency in this group. Ensure criteria for lung volume reduction surgery are considered to improve ppo values and postoperative lung function.
Risk Reduction
Probability for Preoperative Interventions to Reduce the Risk of Pulmonary Complications
| Risk Factor | Intervention | Probability |
|---|---|---|
| Smoking | Cessation >8 weeks | ++++ |
| Cessation <8 weeks | + | |
| Exacerbation of COPD or asthma | Steroids, bronchodilators, and delay elective surgery | ++++ |
| Stable COPD or asthma | Antibiotics indicated by sputum | +++ |
| Physiotherapy | ++++ | |
| Bronchodilators | +++ | |
| Rehabilitation | ++ | |
| Obesity | Physiotherapy | ++++ |
| Weight loss | +++ | |
| Malnutrition | Oral nutrition program | ++ |
++++ = Multiple studies confirming; +++ = both some data plus physiologic rationale supporting; ++ = either some data or good physiologic rationale; + = limited data or physiologic rationale
Post-Thoracotomy Complications
Post-Lobectomy Complications and Hospital Length of Stay (LOS)
| Complication | All Patients (n = 4,979) |
|---|---|
| Pneumonia | 4% |
| Atelectasis | 4% |
| ARDS | 1% |
| Myocardial Infarction | 0.4% |
| Ileus | 1% |
| Renal Failure | 1.4% |
| Pulmonary Embolus | 0.3% |
| Atrial Arrhythmias | 12% |
| Air Leak | 10% |
Links
- Exercise testing and optimization
- Lung function testing
- One lung Ventilation and VATS
- Lung resection
- Long volume reduction surgery
- Post op pulmonary complications
References:
- Slinger, P. and Darling, G. (2011). Preanesthetic assessment for thoracic surgery. Principles and Practice of Anesthesia for Thoracic Surgery, 11-34. https://doi.org/10.1007/978-1-4419-0184-2_2
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resection: 2023 ERS/ESTS consensus update. Eur Respir J. 2023;61:2202431. publications.ersnet.org
- Sentürk M, Ince I, Yoldas T, et al. Pre-operative pulmonary evaluation for lung resection: current concepts. J Thorac Dis. 2021;13:5131-5148. pmc.ncbi.nlm.nih.gov
- British Thoracic Society. Guidelines for the pre-operative assessment of patients undergoing lung resection. Thorax. 2022;77:1157-1179. mdpi.com
- American Thoracic Society. Association of pre-operative lung function with complications after resection for NSCLC. Ann Am Thorac Soc. 2023;20:1012-1022. atsjournals.org
- Wassall H, Dodd J, Hardman J. Cardiopulmonary exercise testing in thoracic surgery. Br J Anaesth Educ. 2024;24:30-36. ncbi.nlm.nih.gov
- Bagshaw SM, West MJA. Evaluation of pre-operative cardiopulmonary reserve and surgical risk in lung cancer resection. Chest. 2024;166:345-356. pmc.ncbi.nlm.nih.gov
- Brunelli A, et al. Integrated cardiopulmonary risk model for lung resection: ERS/ESTS 2023 update. Eur Respir J. 2023;61:2202431.
- Licker M, Karenovics W. Post-operative atrial fibrillation after lung surgery: prevention and treatment. J Thorac Cardiovasc Surg. 2024;168:187-195.
- British Thoracic Society. Peri-operative management of COPD and smoking cessation. Thorax. 2022;77:1190-1202.
- Gajdos C, et al. Frailty and outcomes after thoracic surgery in the elderly. Ann Thorac Surg. 2021;112:1233-1240.
- Khullar OV, et al. Acute kidney injury after lung resection: incidence and predictors. Chest. 2023;163:445-456.
- Sentürk M, et al. One-lung ventilation: physiological considerations and management. Curr Opin Anaesthesiol. 2024;37:15-22.
- The Calgary Guide to Understanding Disease. (2024). Retrieved June 5, 2024, from https://calgaryguide.ucalgary.ca/
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org/
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
Summaries:
CPEX
Thoracic anaesthesia
Pre-op cardiac assessment
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “d8440a56-520a-4b61-b00f-aaabb311d7da”



