- Cardiac Surgery and Blood Conservation
- Links
{}
Cardiac Surgery and Blood Conservation
Why Blood Matters in Cardiac Surgery
- Cardiopulmonary bypass (CPB) produces haemodilution, platelet dysfunction, fibrinolysis and consumption of clotting factors; 15–30 % of patients bleed sufficiently to require allogeneic blood. Transfusion, bleeding and pre-operative anaemia each independently predict death, stroke, renal failure and prolonged ICU stay.
- Key transfusion-risk determinants: advanced age, female sex/small body surface area, pre-op Hb < 12 g dl⁻¹, dual-antiplatelet or anticoagulant therapy, urgent/complex surgery, CPB time > 120 min, re-operation, and low institutional use of patient-blood-management (PBM) bundles.
Anaemia in the Peri-operative Period
| Hb (g dl⁻¹) | Typical physiological state | Clinical guidance |
|---|---|---|
| > 10 | Normal reserve | Transfusion rarely indicated |
| 7–8 | Compensated (↑CO, ↑O₂ extraction) | Restrictive threshold (TRICS-III) safe in haemodynamically stable adults |
| < 6 | Decompensation begins in splanchnic & renal beds | Consider transfusion even if stable |
| < 4 | Cerebral & myocardial hypoxia | Urgent transfusion |
- DO₂ = CO × [1.34 × Hb × SaO₂ + 0.003 × PaO₂]–any fall in Hb must be countered by ↑CO or SaO₂; these compensations are attenuated by β-blockade, LV dysfunction and CPB cooling.
Effects of Anaemia
Physiological Effects
- DO₂ = CO x (Hb x 1.34 x SaO₂) + 0.003PaO₂
- ↓ Hb ⇒ ↓ O₂ carrying capacity → ↓ DO₂ → compensation → decompensation → hypoxia → organ dysfunction
Adaptation to Anaemia
- Non-Hemodynamic
- ↑ EPO ← hypoxia
- ↑ O₂ extraction ← ↓ Hb O₂ affinity ← rightward OHDC ← ↑ 2,3 DPG
- Hemodynamic
- ↓ AL ← ↓ SVR ← ↓ blood viscosity + vasodilation (endothelial NO + hypoxic metabolites)
- ↑ PL ← ↑ venous return ← venoconstriction ← SNS
- ↑ MC ← ↑ PL (Frank-Starling) + SNS
- ⇒ ↑ CO
Haemodynamic Effects Summary
- ↓ Erythrocytes
- ↓ Blood viscosity
- ↓ Peripheral resistance
- ↓ Hemoglobin
- ↓ O₂ delivery
- ↑ Chemoreceptor discharge
- ↑ Sympathetic activity
- ↑ Heart rate
- ↑ Myocardial contractility
- ↑ Venous tone
- Arterial vasodilation
- ↑ EDRF (NO) availability
- Recruitment of vessels (angiogenesis)
- ↑ Flow partition
- ↑ Cardiac output
- ↑ Stroke volume
- ↑ Venous return
- ↓ Resistance to venous return
- ↑ Venous return
- ↑ Stroke volume
- ↑ Cardiac output
- ↑ Flow partition
- Recruitment of vessels (angiogenesis)
- ↑ EDRF (NO) availability
- ↑ Sympathetic activity
- ↑ Chemoreceptor discharge
- ↓ O₂ delivery
Contemporary Transfusion Thresholds
- Restrictive strategy (Hb < 7–7.5 g dl⁻¹) is non-inferior to liberal (Hb < 9–9.5 g dl⁻¹) for death, MI, stroke or AKI (TRICS-III, 7 500 pts).
- Maintain Hb ≥ 8–9 g dl⁻¹ if active ischaemia, ECMO, severe pulmonary hypertension or pregnancy.
- Paediatric thresholds are higher and age-specific
STS/SCA/AmSECT/SABM 2021 PBM Guideline – Condensed Highlights
| Class | Recommendation (adult cardiac surgery) |
|---|---|
| I | • Screen & treat anaemia ≥ 4 weeks pre-op (iv iron ± erythropoietin) • All patients receive intra-operative antifibrinolytic (tranexamic acid preferred) • Point-of-care (POC) coagulation testing (TEG/ROTEM) to guide component therapy • Cell-salvage & retrograde autologous priming (RAP) for on-pump cases |
| IIa | • Stop P2Y₁₂ inhibitor ≥ 5 days (clopidogrel) or ≥ 7 days (ticagrelor, prasugrel) pre-CPB if elective • Acute normovolaemic haemodilution (ANH) when pre-op Hb > 13 g dl⁻¹ • Mini-CPB circuits, centrifugal pumps, leukocyte-reduced filters |
| IIb | • Topical TXA/fibrin sealants in re-do sternotomy • Prothrombin-complex concentrate (PCC) for refractory coagulopathy when fibrinogen ≥ 2 g l⁻¹ |
| III | • Prophylactic DDAVP or rFVIIa; routine transfusion when Hb > 10 g dl⁻¹ |
Blood-saving Techniques
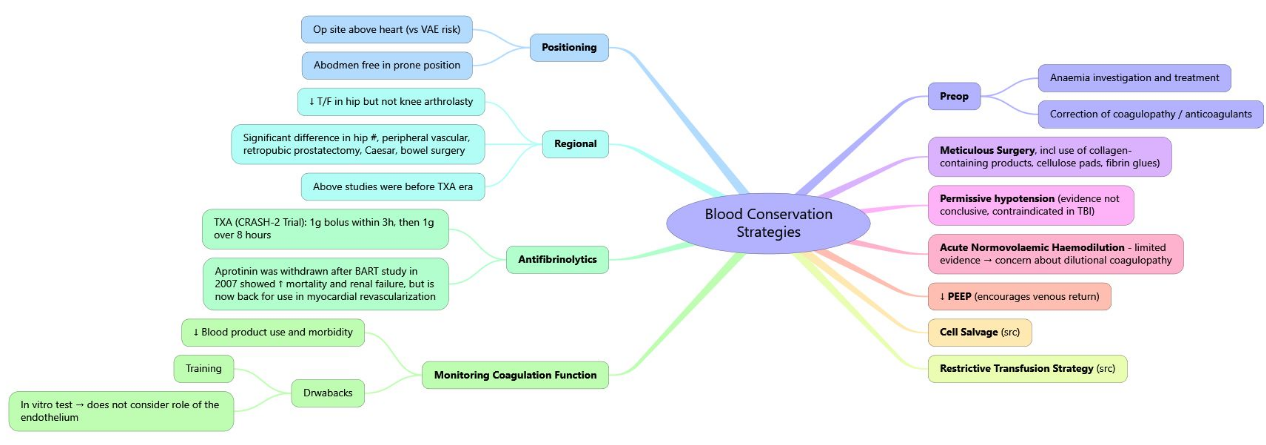
| Technique (timing) | Practical notes | Evidence summary |
|---|---|---|
| Cell-salvage (intra/post-CPB) | Heparinised shed blood washed & returned | Reduces allogeneic RBC ~ 1 unit/pt; no ↑ stroke/AKI |
| Acute normovolaemic haemodilution (induction) | 400–800 ml removed into CPDA, replaced with crystalloid/colloid; reinfuse after protamine | ↓ blood loss 10–20 %, ↓ platelet transfusion |
| Retrograde autologous priming (before CPB) | Displaces crystalloid prime with patient’s blood (≈ 250 ml) | Small ↓ RBC use; minimal haemodynamic impact |
| Minior vacuum-assisted venous-drain CPB | Circuit volume ≤ 1000 ml, reduced surfaces | Meta-analysis: 25 % fewer transfusions, shorter ventilation time |
| Visco-elastic POC testing | TEG/ROTEM at separation & in ICU | 30–40 % reduction in platelets/FFP; cost-effective |
| Topical fibrin/TXA | Spray/foam to sternal edges & graft sites | ↓ chest-tube loss ~ 200 ml; no systemic thrombosis |
Erythropoiesis-stimulating & Iron Therapy
- iv iron ± ferric carboxymaltose 1000 mg preferred for functional/absolute iron deficiency (TSAT < 20 %).
- Erythropoietin-α 40 000 IU SC weekly × 3–4 weeks (or 600 IU kg⁻¹) reduces transfusion by ~ 40 % in elective CABG, but benefits must be weighed against thrombotic risk and cost.
- Aim Hb > 13 g dl⁻¹ on day of surgery.
Pre-operative Autologous Donation (PAD)
Rarely used today owing to logistic burden and iron depletion; appropriate only in young, low-risk patients undergoing delayed, high-blood-loss procedures where PBM alternatives unavailable.
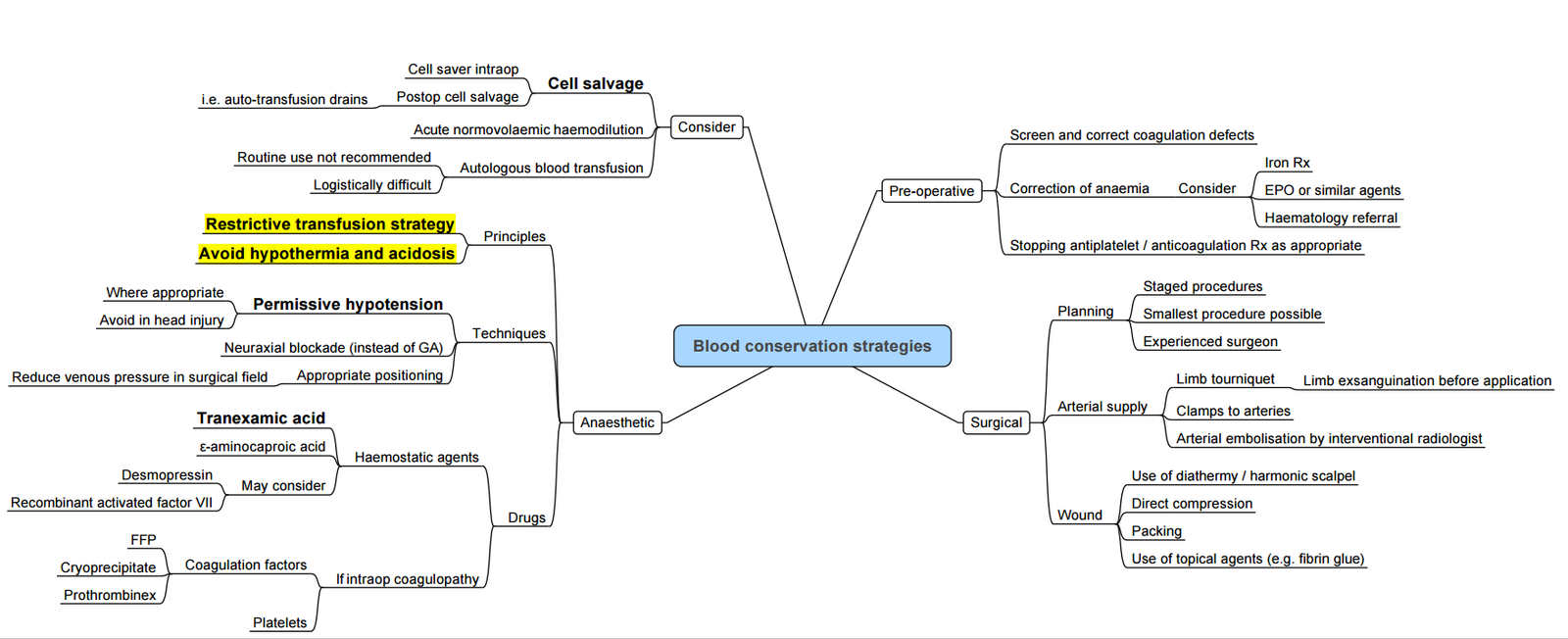
Pharmacological Haemostasis
| Agent | Dose (adult) | Key safety signals |
|---|---|---|
| Tranexamic acid (TXA) | 15–30 mg kg⁻¹ IV load plus 2 mg kg⁻¹ in pump prime then 1 mg kg⁻¹ h⁻¹ | Seizures if total > 100 mg kg⁻¹ or rapid bolus; no ↑ MI/stroke in modern RCTs |
| ε-Aminocaproic acid | 100 mg kg⁻¹ load ⇒ 10 mg kg⁻¹ h⁻¹ | Less potent; minimal seizure risk |
| Aprotinin | Re-introduced in EU (2023) for high-risk re-do cases; strict renal monitoring | Contra-indicated if previous aprotinin exposure within 12 mths |
| Desmopressin (DDAVP) | 0.3 µg kg⁻¹ over 15 min for confirmed platelet-storage-pool disorder or vWD type 1 | Facial flushing, hyponatraemia |
| PCC (4-factor) | 25–50 IU kg⁻¹ if INR > 1.8 and active bleeding | Thrombosis if fibrinogen < 1.5 g l⁻¹ |
- Recombinant factor VIIa reserved for life-threatening bleeding after failure of all other measures; stroke risk ~ 1–2 %.
Red-cell Storage Lesions
- Large RCTs in ICU (ABLE, 2015) and cardiac surgery (RECESS, 2015) showed no difference in mortality or MODS with 1-week-old versus standard-issue (median 21 days) RBC.
- Observational signals linking > 28-day units to AKI and infection persist, but guidelines do not mandate “fresh” blood except for neonates, massive exchange or haemoglobinopathies.
- Main biochemical changes (≥ 21 days):
- ↑ K⁺, free Hb, lactate, micro-vesicles
- ↓ pH, 2,3-DPG, ATP, S-nitrosylated Hb
- ⇔ Cl⁻, Ca²⁺, Mg²⁺, metHb
- Most changes reverse within 24 h in vivo; clinical impact appears minimal
Take-home Algorithm (adult On-pump CABG)
- ≥ 4 weeks pre-op–full blood count, ferritin/TSAT; treat iron-deficiency ± EPO.
- < 7 days–stop clopidogrel/ticagrelor; continue aspirin unless very low bleeding-risk surgery.
- Induction–ANH if Hb > 13; give TXA bolus.
- CPB–RAP, cell saver, mini-circuit, maintain ACT > 450 s.
- Separation–VET-guided reversal (protamine), fibrinogen concentrate if FIBTEM < 8 mm.
- ICU–Restrictive transfusion unless shock/ischaemia; reinfuse mediastinal cell-salvage; early extubation.
Links
- Blood transfusions and conservation strategies
- Haematology and Blood testing
- Jehovah’s witness
- Cardiac surgery
References:
1. Utley, J. R., Moores, W. Y., & Stephens, D. (1981). Blood conservation techniques. The Annals of Thoracic Surgery, 31(5), 482-490. https://doi.org/10.1016/s0003-4975(10)61007-7
2. Wise, R., Bishop, D. G., Gibbs, M., Govender, K., James, M. F., Kabambi, F., … & Turton, E. (2020). South african society of anaesthesiologists perioperative patient blood management guidelines 2020. Southern African Journal of Anaesthesia and Analgesia, S1-S68. https://doi.org/10.36303/sajaa.2020.26.6.s1
3. Pagano D, Milojevic M, Meesters MI, _et al._ 2021 STS/SCA/AmSECT/SABM update to the clinical practice guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth. 2021;35(10):3073-131. https://doi.org/10.1053/j.jvca.2021.04.012
4. Mazer CD, Whitlock RP, Fergusson DA, _et al._ Restrictive or liberal red-cell transfusion for cardiac surgery (TRICS-III). N Engl J Med. 2017;377:2133-44.
5. Mazer CD, et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N Engl J Med. 2018;378:1289-98.
6. Weber CF, Hildebrand W, Lobmeyer MT, _et al._ High-dose tranexamic acid and postoperative seizures in cardiac surgery. Anesthesiology. 2022;136:186-98.
7. Kirsch ME, Joly H, Barbier F, _et al._ Effect of acute normovolemic hemodilution on transfusion requirements in adult cardiac surgery: systematic review & meta-analysis. Br J Anaesth. 2020;124:718-28.
8. Lacroix J, et al. Age of transfused blood in critically ill adults (ABLE). N Engl J Med. 2015;372:1410-18.
9. Steiner ME, et al. Effects of red-cell storage duration on cardiac surgery outcomes (RECESS). N Engl J Med. 2015;372:1419-29.
10. Görlinger K, Shore-Lesserson L, Dirkmann D, _et al._ Management of hemorrhage in cardiac surgery–perioperative bleeding management consensus. BJA. 2022;129:899-918.
11. Sharma R, Huang Y, Dizdarevic A. Blood Conservation Techniques and Strategies in Orthopedic Anesthesia Practice. Anesthesiol Clin. 2022 Sep;40(3):511-527. doi: 10.1016/j.anclin.2022.06.002. Epub 2022 Jul 12. PMID: 36049878.
Summaries
Autologous blood transfusion
Blood conservation- video
Copyright**
© 2025 Francois Uys. All Rights Reserved.
id: “ba9ff221-3295-491d-8f3f-163625c8b2ff”



