{}
Inotropes & Vasopressors in Shock
Haemodynamic Targets in Shock
| Shock phenotype | Primary derangement | First‐line pharmacology | Focus of therapy |
|---|---|---|---|
| Cardiogenic (CS) | ↓ Stroke volume / cardiac output | Norepinephrine (restore MAP) ± Dobutamine or Milrinone (augment CO) | Maintain coronary perfusion pressure, improve contractility |
| Distributive (septic, anaphylactic, neurogenic) | ↓ Systemic vascular resistance (SVR) | Norepinephrine (add Vasopressin when NE ≈ 0.25–0.5 µg kg⁻¹ min⁻¹; ± Epinephrine or Angiotensin II in refractory cases) | Reverse vasoplegia |
| Hypovolaemic | ↓ Pre-load | Balanced crystalloids ± blood products; vasopressor (NE) only when hypotension persists after fluid loading | Volume replacement |
| Obstructive | Mechanical obstruction to filling/emptying | Immediate source control (pericardiocentesis, thrombolysis, chest drain); vasopressor/inotrope bridge (NE ± Dobutamine) | Relieve obstruction |
Pearl: _Norepinephrine is now the front-line vasopressor for both septic and cardiogenic shock; dopamine is reserved only for selected patients with profound bradycardia and low arrhythmic risk.
Pharmacological Classes
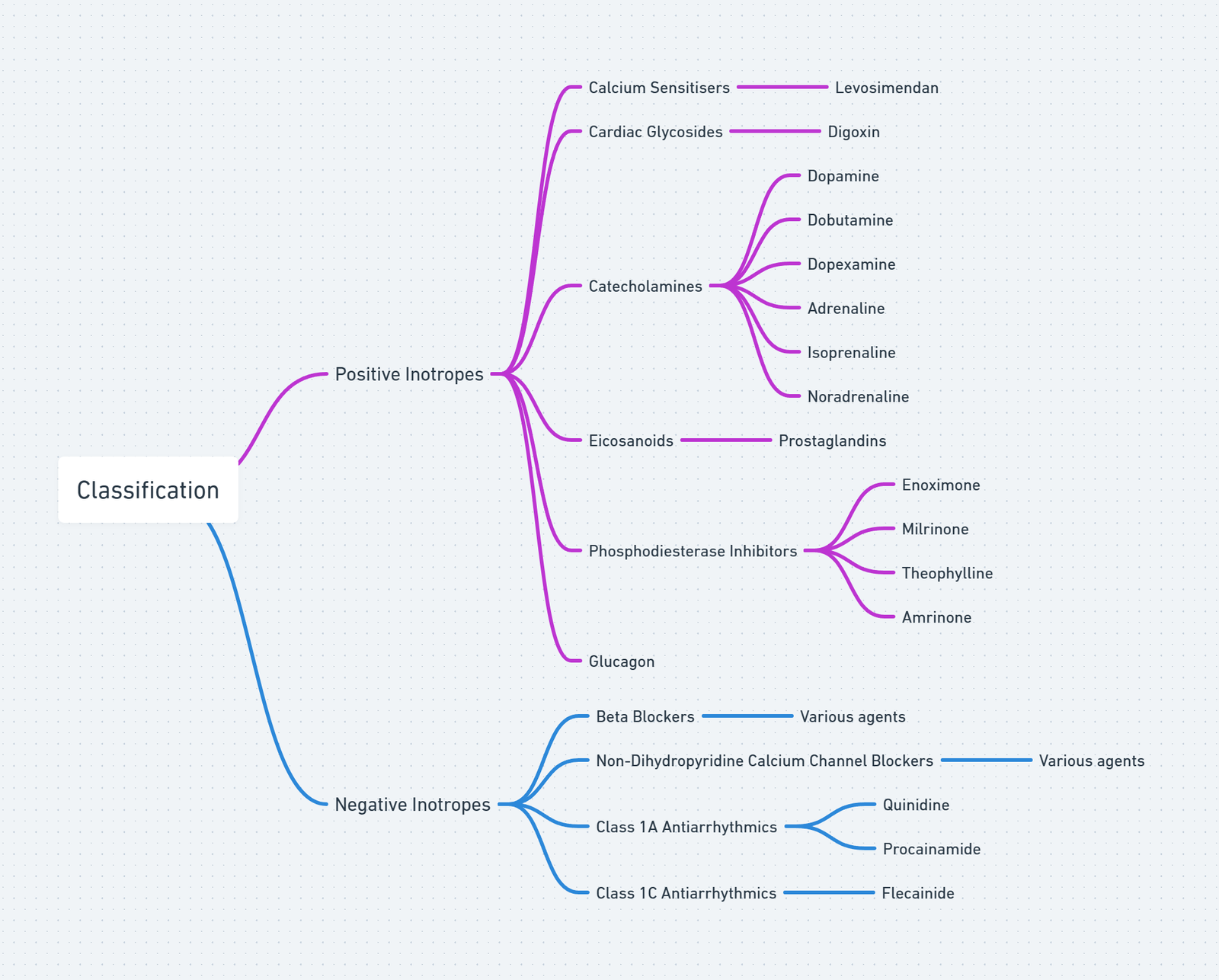
View or edit this diagram in Whimsical.
Adrenergic Catecholamines
| Drug | Dominant receptors | Typical infusion (adult) | Major clinical use | Key cautions |
|---|---|---|---|---|
| Norepinephrine | α₁ +++ | 0.05–1 µg kg⁻¹ min⁻¹ | First-line vasopressor in septic & cardiogenic shock | Digital ischaemia, ↑ after-load in LV failure |
| Epinephrine | α₁ ++ β₁ ++ β₂ + | 0.03–0.5 µg kg⁻¹ min⁻¹ | Add-on in septic shock, anaphylaxis | Tachyarrhythmia, ↑ lactate (glycolysis) |
| Dobutamine | β₁ +++ β₂ + | 2–20 µg kg⁻¹ min⁻¹ | Inodilator for CS with adequate BP | AF/flutter acceleration, tachyphylaxis |
| Dopamine | β₁ + (α at >10 µg kg⁻¹ min⁻¹) | 2–20 µg kg⁻¹ min⁻¹ | Very limited role (bradycardic CS) | ↑ Mortality & arrhythmia v NE in septic shock pubmed.ncbi.nlm.nih.gov |
| Phenylephrine | α₁ +++ | 0.5–5 µg kg⁻¹ min⁻¹ | Salvage vasopressor when tachy-dysrhythmia precludes NE | Reflex bradycardia, ↓ stroke volume |
Non-adrenergic Vasopressors
| Drug | Dose | Comments |
|---|---|---|
| Vasopressin | Fixed 0.03 units min⁻¹ | Add to NE once >0.25 µg kg⁻¹ min⁻¹ (base) to spare catecholamine need ccforum.biomedcentral.com |
| Angiotensin II | 10–40 ng kg⁻¹ min⁻¹ | Raises MAP in catecholamine-refractory vasoplegia (ATHOS-3); mortality neutral pmc.ncbi.nlm.nih.gov |
Inodilators & Calcium Sensitisers
| Agent | Mechanism | Usual range | Evidence highlights |
|---|---|---|---|
| Milrinone | PDE-3 inhibition → ↑ cAMP | 0.125–0.75 µg kg⁻¹ min⁻¹ (renal dose ↓) | DOREMI RCT: no outcome difference v dobutamine in CS thebottomline.org.uk |
| Levosimendan | Ca²⁺ sensitisation + K_ATP opening | 12.5 µg kg⁻¹ load over 10 min, then 0.1 µg kg⁻¹ min⁻¹ × 24 h | Meta-analysis 2024: ↓ mortality & LCOS in high-risk CABG pubmed.ncbi.nlm.nih.gov |
Miscellaneous
| Drug | Role |
|---|---|
| Digoxin | Rate control in AF with HF; weak positive inotrope |
| Isoproterenol | Chemical pacing in severe bradycardia or heart transplant |
| Ephedrine (bolus) | Indirect sympathomimetic for transient anaesthesia-related hypotension |
Receptors
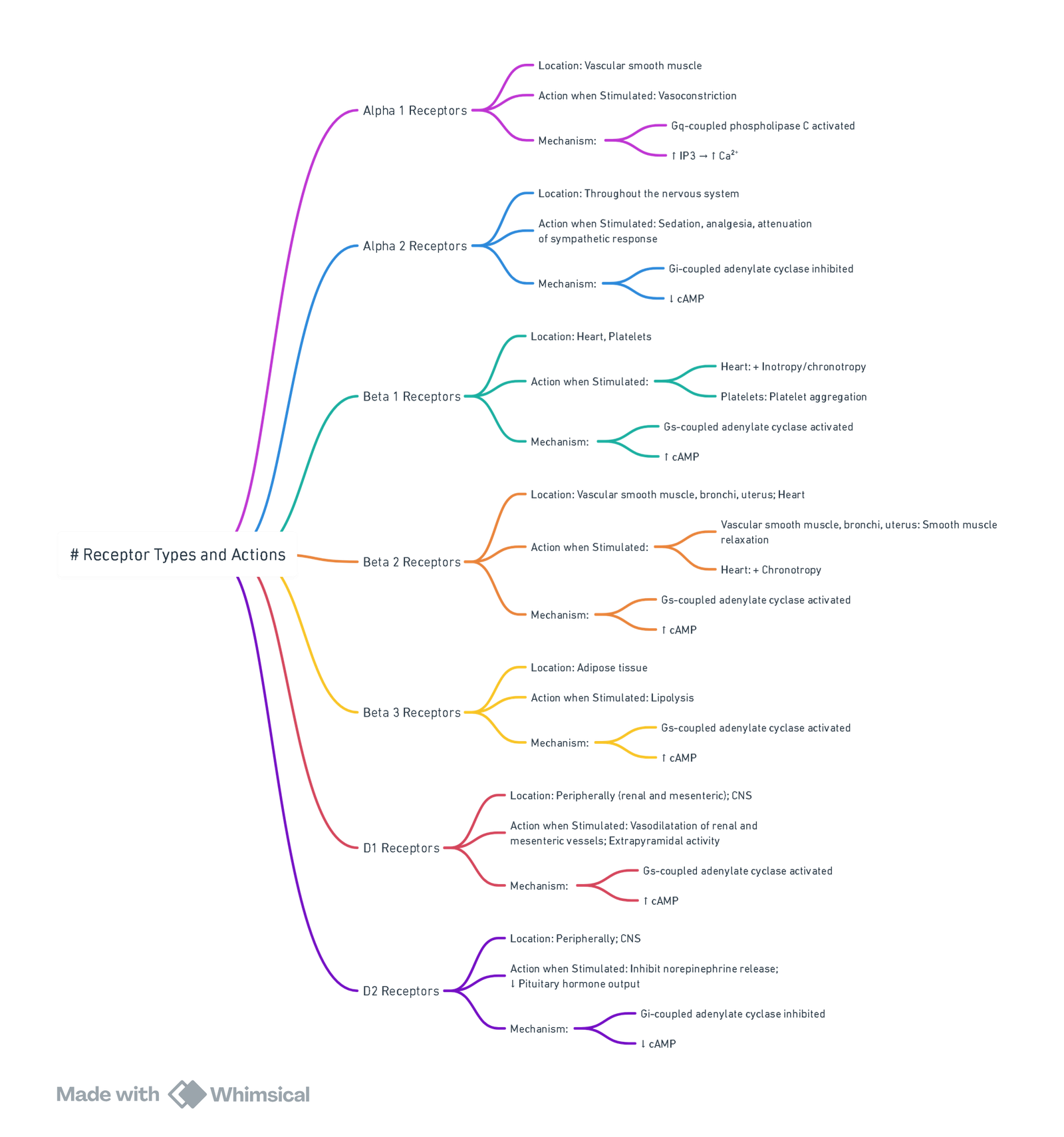
View or edit this diagram in Whimsical.
Guideline‐driven Strategies
Septic Shock (SSC 2021)
- Balanced crystalloid 30 mL kg⁻¹ within first 3 h.
- Norepinephrine to target MAP ≥ 65 mmHg.
- Add Vasopressin at NE 0.25–0.5 µg kg⁻¹ min⁻¹.
- Epinephrine or Angiotensin II for refractory hypotension.
Cardiogenic Shock (ESC/AHA 2022–24)
- Norepinephrine preferred vasopressor when SBP < 90 mmHg.
- Dobutamine first-choice inotrope; switch to Milrinone if on β-blockers or with pulmonary hypertension.
- No survival difference between milrinone and dobutamine (DOREMI), choose based on renal function, arrhythmia risk and cost.
Post-cardiotomy Low-output Syndrome
- Consider Levosimendan loading before weaning from CPB in patients with LVEF < 35 %.
Practical Dosing & Monitoring Tips
- Dilution: NE 4 mg in 250 mL (16 µg mL⁻¹); Epi 1 mg/100 mL (10 µg mL⁻¹).
- Titration: Adjust q2–5 min aiming for predefined MAP/CI; avoid escalating NE > 1 µg kg⁻¹ min⁻¹ without adding a second agent.
- Lines: Central venous access strongly recommended; peripheral NE safe for < 6 h via 18 G proximal forearm/antecubital vein.
- End-organ markers: serum lactate trend, urine output, ScvO₂, arterial pulse pressure variation.
- Weaning: Reduce vasopressin last to avoid rebound hypotension.
Adverse Effects & Mitigation
| Class | Common complications | Prevention |
|---|---|---|
| Adrenergic catecholamines | Tachyarrhythmia, myocardial ischaemia, hyperlactataemia | Correct hypoxia/acid–base, monitor ECG & lactate |
| Vasopressin | Digital or splanchnic ischaemia, hyponatraemia | Limit to 0.03–0.04 units min⁻¹ |
| PDE-3 inhibitors | Hypotension, ventricular arrhythmia | Use with invasive arterial line; reduce dose in eGFR < 50 mL min⁻¹ |
| Levosimendan | Headache, hypotension, hypokalaemia | Pre-load optimisation, monitor K⁺ |
Different Types of Drugs
Doses and Characteristics
| Agent | Initial dose | Usual maintenance dose range | Range of maximum doses used in refractory shock | Role in therapy and selected characteristics |
|---|---|---|---|---|
| Vasopressors (alpha-1 adrenergic) | ||||
| Norepinephrine (noradrenaline) | 5 to 15 mcg/minute (0.05 to 0.15 mcg/kg/minute) | 2 to 80 mcg/minute (0.025 to 1 mcg/kg/minute) | 80 to 250 mcg/minute (1 to 3.3 mcg/kg/minute) | Initial vasopressor of choice in septic, cardiogenic, and hypovolemic shock. Wide range of doses utilized. Must be diluted (e.g., usual concentration is 4 mg in 250 mL of D5W or NS for mcg/min programs). |
| Epinephrine (adrenaline) | 1 to 15 mcg/minute (0.01 to 0.2 mcg/kg/minute) | 1 to 10 mcg/minute (0.01 to 0.5 mcg/kg/minute) | 40 to 160 mcg/minute (0.5 to 2 mcg/kg/minute) | Initial vasopressor of choice in anaphylactic shock. Typically an add-on vasopressor in septic shock when an additional agent is required to raise MAP or target and occasionally an alternative first-line agent if norepinephrine is contraindicated. Increases heart rate; may induce tachyarrhythmias and ischemia. For inotropy, doses in the higher end of the suggested range is needed. Elevates lactate concentrations during initial administration (e.g., may preclude use of lactate clearance goal); may decrease mesenteric perfusion. Must be diluted (e.g., usual concentration is 1 mg in 250 mL of D5W or NS for mcg/min programs). |
| Phenylephrine | 40 to 160 mcg/minute until stabilized (alternatively, 0.5 to 2 mcg/kg/minute) | 20 to 400 mcg/minute (0.25 to 5 mcg/kg/minute) | 8 to 730 mcg/minute (0.1 to 9.1 mcg/kg/minute) | Pure alpha adrenergic vasoconstrictor. May be considered when tachyarrhythmias preclude use of norepinephrine. Alternative vasopressor for patients with septic shock with (1) adequate intravascular volume (2) mild to moderate hypotension, and (3) low risk for tachyarrhythmias. Less effective than norepinephrine for raising MAP in septic shock. Lower doses (e.g., 1 to 3 mcg/kg/minute) should not be used for protective effect and can cause hypotension during weaning. Must be diluted (e.g., usual concentration is 10 mg in 250 mL D5W or NS [40 mcg/mL] or 100 mg in 250 mL D5W or NS [400 mcg/mL]); use of commercially available pre-diluted solution is preferred. |
| Dopamine | 2 to 5 mcg/kg/minute | 2 to 20 mcg/kg/minute | 20 mcg/kg/minute | Alternative to norepinephrine in septic shock in highly selected patients (e.g., with absolute or relative bradycardia and a low risk of tachyarrhythmias). More adverse effects (e.g., tachycardia, arrhythmias) particularly at doses >20 mcg/kg/minute and less effective than norepinephrine for reversing hypotension in septic shock. Lower doses (e.g., 1 to 3 mcg/kg/minute) should not be used for protective effect and can cause hypotension during weaning. Must be diluted (e.g., usual concentration is 400 mg in 250 mL D5W [1.6 mg/mL] or 800 mg in 250 mL D5W [3.2 mg/mL]); use of commercially available pre-diluted solution is preferred. |
| Antidiuretic hormone | ||||
| Vasopressin (arginine vasopressin) | 0.03 units/minute | 0.01 to 0.04 units/minute (not titrated) | Doses >0.04 units/minute can cause cardiac ischemia and should be reserved for salvage therapy | Add-on to norepinephrine to raise blood pressure to target MAP or decrease norepinephrine requirement. Not recommended as a replacement for first-line vasopressor. Pure vasoconstrictor; may decrease stroke volume and cardiac output in myocardial dysfunction or precipitate ischemia in coronary artery disease. Must be diluted (e.g., usual concentration is 20 units in 250 mL D5W or NS [0.1 units/mL]). |
| Inotrope (nonadrenergic, PDE3 inhibitor) | ||||
| Milrinone | 0.125 to 0.25 mcg/kg/minute | 0.125 to 0.75 mcg/kg/minute | 0.75 mcg/kg/minute | Alternative for short-term cardiac output augmentation to maintain organ perfusion in cardiogenic shock refractory to other agents. Increases cardiac contractility and modestly increases heart rate at high doses. May cause peripheral vasodilation, hypotension, and/or ventricular arrhythmias. Renally cleared; dose adjustment in renal impairment needed. Must be diluted (e.g., usual concentration is 40 mg in 200 mL D5W [200 mcg/mL]); use of commercially available pre-diluted solution is preferred. |
Catecholamines and Their Cardiac Effects
| Catecholamine Type | Drug | α | β1 | β2 | Mechanism of Action | Cardiac Output | Heart Rate | Dysrhythmias | Peripheral Vascular Resistance | Renal Blood Flow | Mean Arterial Pressure | Airway Resistance | Central Nervous System Stimulation | Single Intravenous Dose (70-kg Adult) | Continuous Infusion Dose (70-kg Adult) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Natural catecholamines | Epinephrine | + | ++ | ++ | Direct | ++ | ++ | +++ | ± | — | + | — | Yes | 2–8 µg | 1–20 µg/min |
| Norepinephrine | +++ | +++ | + | Direct | – | – | + | +++ | — | +++ | NC | No | Not used | 4–16 µg/min | |
| Dopamine | ++ | + | + | Direct | +++ | + | + | + | +++ | + | NC | No | Not used | 2–20 µg/kg/min | |
| Synthetic catecholamines | Isoproterenol | 0 | +++ | +++ | Direct | +++ | +++ | +++ | — | – | ± | — | Yes | 1–4 µg | 1–5 µg/min |
| Dobutamine | 0 | +++ | + | Direct | +++ | + | ± | NC | ++ | + | NC | No | Not used | 2–10 µg/kg/min | |
| Synthetic noncatecholamines | Ephedrine | ++ | + | + | Direct and indirect | ++ | ++ | + | + | — | ++ | — | Yes | 10–25 µg | Not used |
| Phenylephrine | +++ | 0 | + | Direct | – | – | NC | +++ | — | +++ | — | No | 50–100 µg | 20–50 µg/min |
Legend:
- NC: No Change
- –: No significant effect
- ±: Mild effect (positive or negative)
- +: Mild positive effect
- ++: Moderate positive effect
- +++: Strong positive effect
- —: Strong negative effect
Naturally Occurring
Dopamine
- Acts both directly and indirectly.
- The absence of functional groups on the ethylamine sidechain allows it to enter sympathetic nerve terminals and displace noradrenaline from storage vesicles, causing an adrenergic effect, as long as noradrenaline stores have not been depleted.
- Direct function (at doses around 5 µg/kg/min) is via binding to dopamine receptors. Dopamine’s structure does not give it great affinity for α and β receptors, but at doses up to 10-20 µg/kg/min, β1 receptors may be stimulated, causing increased heart rate, contractility, and cardiac output.
- At even higher doses (>20 µg/kg/min), α effects predominate, with peripheral vasoconstriction and increased systemic vascular resistance and venous return.
- Dopamine is described as a general inotrope-vasopressor, but its wide and unpredictable dosage range, as well as reliance on indirect mechanisms of action, usually make it a suboptimal choice of inotrope. It is a potent emetogenic, suppresses prolactin release (impairing immunity) and TSH release.
- The “renoprotective” benefit of dopamine has been disproven, despite its continued use in certain centers. Urine output in these patients likely increased due to the diuretic effect of dopamine (inhibiting renal tubular reabsorption of sodium), rather than improved renal perfusion. Dopamine may cause maldistribution of blood flow from the renal medulla to the cortex and may worsen renal outcomes.
Noradrenaline
- Differs from dopamine by the addition of a single hydroxyl group on the ethylamine sidechain, making it a direct-acting drug with a high affinity for α receptors and moderate affinity for β1 receptors, without much β2 effect.
- This makes it a potent vasoconstrictor via α1 agonism (and lack of β2 vasodilatation) as well as a mild inotrope via a moderate β1 effect.
- It is the agent of choice in states of distributive shock, such as the systemic inflammatory response syndrome (SIRS) or sepsis. It is available in South Africa but can be complicated to obtain for routine clinical use.
Adrenaline
- Formed from noradrenaline, with the addition of a methyl group on the terminal amine, greatly increasing affinity for both β1 and β2 receptors.
- At lower doses it acts predominantly as a β agonist and inotrope, with some vasopressor action. At higher doses (around 1 µg/kg/min) the α1 vasopressor activity dominates, but is not as potent as noradrenaline, due to the offset vasodilation via β2 activity.
- Adrenaline is readily available and in common use globally. Its positive chronotropic and inotropic action increases cardiac workload, and together with additional arrhythmogenicity, it may put patients with ischemic heart disease at risk.
- Plasma glucose is raised by stimulating glycogenolysis, lipolysis, and gluconeogenesis, and may also be affected by changes in insulin secretion (increased by β2, but overridden by α effects).
- Although it is a significant vasopressor, lactate rise is usually due to increased glycolytic flux rather than vasoconstriction and anaerobic metabolism.
- Adrenaline is recommended at a dose of 1 mg every 2-4 minutes as part of the resuscitation guidelines in cardiac arrest, functioning as a pure vasopressor intended to direct blood supply to vital organs.
Synthetic
Isoprenaline
- It is a pure β agonist and was favored for its chronotropy (useful in chemical pacing of bradycardias and denervated hearts) and inotrope-vasodilator action (useful postoperatively in pediatric cardiac patients unable to tolerate increased afterload).
Dobutamine
- Resembles dopamine but has a large hydrocarbon tail which ensures it is direct-acting and increases β receptor selectivity. It was designed to be a pure β agonist and has been termed an “inodilator” due to the inotropic effect at β1 receptors, combined with the afterload-reducing vasodilatory effect of β2 receptor stimulation in the skeletal muscle vascular beds.
- It also increases atrioventricular conduction and may precipitate arrhythmias or increase the ventricular response rate in patients with atrial fibrillation or flutter. It is used in the treatment of low cardiac output states in heart failure and cardiac surgery, at a dose range of 1–20 µg/kg/min. It can also be used for cardiac stress testing as an alternative to exercise.
Phenylephrine
- Is not a catecholamine, due to the loss of a hydroxyl group from the benzene ring, but otherwise looks identical to adrenaline.
- This relatively minor structural change prevents it from binding at β receptors, and it is a pure α agonist, causing an increase in systemic vascular resistance and blood pressure.
- It is far less potent than adrenaline and must be given in 10-fold higher doses (50–100 µg boluses intravenously).
- Degradation by COMT is also less effective, meaning the duration of action is longer. It should be used with caution, or not at all, in the setting of relative bradycardia or decreased inotropic states, where a β agonist should be considered to avoid precipitating acute cardiac failure.
Ephedrine
- Is a naturally occurring stimulant in Ephedra plants, but is manufactured synthetically for clinical use.
- The absence of hydroxyl groups means that it is lipid-soluble enough to enter neurons and act indirectly by releasing noradrenaline, its major mechanism of action. It is far less potent as a direct agonist, and 1000-fold doses are required, also leading to rapid depletion of stored noradrenaline.
- It is not degraded by MAO or COMT and therefore has a longer duration of action.
- Ephedrine causes a mild increase in inotropy and vasoconstriction when given in intravenous boluses of 5–10 mg.
- It may become ineffective after repeated doses or in patients in whom noradrenaline stores are depleted.
Amphetamines
- Also have a benzene ring with no hydroxyl group, are highly lipid-soluble and penetrate the blood-brain barrier with ease.
- Although they have no direct adrenoreceptor effect, they displace catecholamines from neuronal storage vesicles and can cause central release of large amounts of catecholamines. Originally developed as medication for appetite suppression and mood disorder, they have subsequently mostly become drugs of abuse.
Phosphodiesterase (PDE) Inhibitors
- Positive inotropes that are not dependent on adrenoceptor activation, as they increase the intracellular action of cAMP and cyclic guanosine monophosphate (cGMP) by preventing their degradation.
- This may be particularly useful in cardiac failure when downregulation of β receptors has occurred.
- The selective PDE III inhibitor milrinone allows accumulation of cAMP in the cardiac myocyte, increasing cardiac contractility, enhancing left ventricular relaxation, and improving early ventricular filling.
- Conversely, in smooth muscle, increased cAMP prevents calcium release and promotes smooth muscle relaxation and reduced peripheral and pulmonary vascular resistance.
Digoxin
- Causes a modest increase in contractility by reversibly binding to Na+-K+-ATPase in the cardiac myocyte, which leads to increased availability of intracellular calcium and increased contractility.
Levosimendan
- Is a myocardial calcium sensitizer and inodilator, which improves contractility without increasing intracellular calcium or cAMP, and thereby doesn’t increase myocardial oxygen demand.
Links
References:
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181-1247. pmc.ncbi.nlm.nih.gov
- Guerci P, Belveyre T, Mongardon N, et al. When to start vasopressin in septic shock: the strategy we propose. Crit Care. 2022;26:125. ccforum.biomedcentral.com
- Xu QY, Jin YH, Li Y, et al. Application of norepinephrine in the treatment of septic shock: a meta-analysis. Ir J Med Sci. 2025;194:361-369. link.springer.com
- Møller JE, Jentzer JC, Thiele H, et al. Management of cardiogenic shock: state-of-the-art 2024. Intensive Care Med. 2024;50:1189-1210. link.springer.com
- Parikh KD, Collet-Flesch L, et al. Milrinone as compared with dobutamine in the treatment of cardiogenic shock (DOREMI). N Engl J Med. 2021;385:516-525. thebottomline.org.uk
- Xu X, Zhang C, et al. Clinical outcomes of angiotensin II therapy in vasoplegic shock: a systematic review and meta-analysis. Crit Care. 2024;28:102. pmc.ncbi.nlm.nih.gov
- Li B, Zhao J, et al. Pre-operative levosimendan in severe LV dysfunction undergoing CABG: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2024;38:455-463. pubmed.ncbi.nlm.nih.gov
- The Calgary Guide to Understanding Disease. (2024). Retrieved June 5, 2024, from https://calgaryguide.ucalgary.ca/
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org/
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
- ICU One Pager. (2024). Retrieved June 5, 2024, from https://onepagericu.com/
Summaries:
Vasopressors
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “1f758fc9-8ad6-4e9f-9b4d-beae72a91cf0”



