- Cardiac Transplant
- Contraindications
- Donor Management (heart donors)
- Physiology of the Transplanted (Denervated) Heart
- Immunosuppression & Major Complications
- Perioperative Management of Orthotopic Heart Transplantation (OHT)
- Aims and Guiding Principles
- Pre-induction Checklist (recipient)
- Induction & Maintenance
- CPB Conduct (high-level)
- Reperfusion and Separation from CPB
- Primary Graft Dysfunction (PGD—Heart)
- Vasoplegic Syndrome after CPB (including during OHT)
- Haemostasis and Transfusion
- Early Post-operative Management (ICU)
- Special Situations
- Fast Reference (doses & targets)
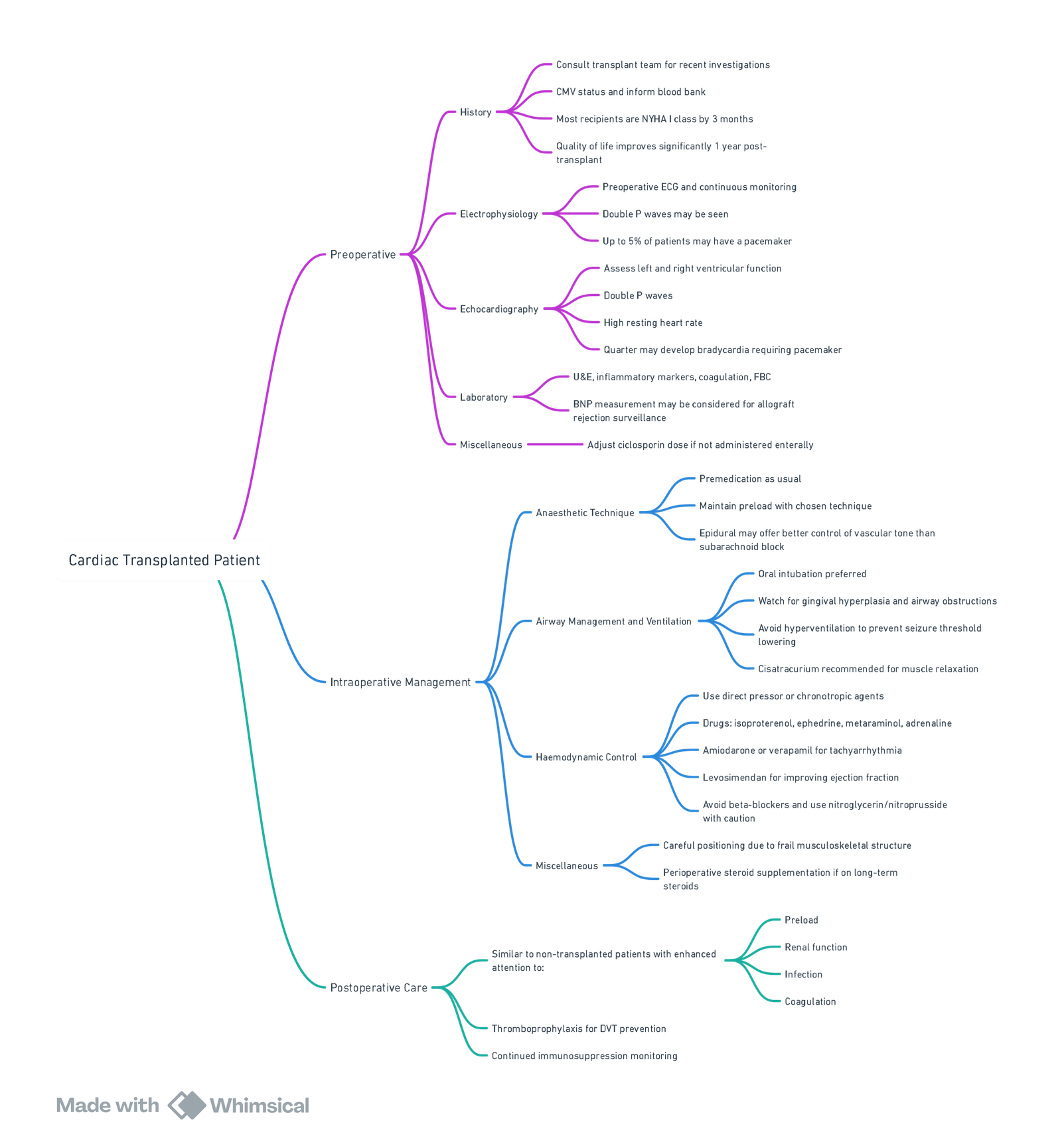
- Anaesthesia for Cardiac Transplant Recipients
- Anaesthetic Concerns in Recipients of Heart Transplants Presenting for Non-Transplant Surgery
- Lung Transplant
- Criteria for Listing for Lung Transplantation for Different Underlying Lung Pathologies
- Criteria for Listing for Lung Transplantation (by Underlying pathology)
- Contraindications to Lung Transplantation
- Conduct of Anaesthesia for Lung Transplant
- Cardiopulmonary Bypass (CPB) Vs ECMO — Practical pros/cons
- Primary Graft Dysfunction (PGD)
- Anaesthetic Concerns in Recipients Presenting for Non-transplant Surgery
- Links
{}
Cardiac Transplant
Contraindications
- Core principle: list only when predicted post-transplant survival is acceptable and short-term benefit outweighs wait-list and peri-operative risks.
Absolute (typical)
- Active, uncontrolled infection (including sepsis).
- Active malignancy with high recurrence risk (tumour-specific disease-free intervals apply).
- Irreversible end-organ dysfunction not amenable to combined transplant (e.g., advanced chronic kidney disease unless kidney–heart considered).
- Fixed, prohibitive pulmonary hypertension despite vasodilator testing (e.g., transpulmonary gradient >15–20 mmHg and/or PVR >3–5 WU), for isolated heart transplant.
- Non-adherence, active substance use, or inability to comply with complex immunosuppression/monitoring.
- Predicted 1-year post-transplant survival <50% using centre-adopted risk models.
Relative / Context-dependent
- Advanced age (no absolute upper limit; outcomes decline >70–75 years).
- Obesity (BMI ≥35 kg m⁻² unfavourable), frailty, severe osteoporosis.
- Significant peripheral/cerebrovascular disease or coronary disease not amenable to revascularisation.
- HIV/HBV/HCV if uncontrolled; highly sensitised recipients (high cPRA).
- Refusal of blood products.
Donor Management (heart donors)
Donor Management Goals (DMGs)
Aim to meet standardised ICU targets before procurement; achieving DMGs increases organ utilisation:
- MAP 65–90 mmHg (prefer noradrenaline ± vasopressin).
- CVP 4–10 mmHg; cardiac index >2.4 L min⁻¹ m⁻²; urine output >1 mL kg⁻¹ h⁻¹.
- SpO₂ ≥95% with PaO₂/FiO₂ >300; pH 7.35–7.45; normothermia 36–37.5 °C.
- Hb ≥10 g dL⁻¹ (Hct ≥30%).
- Glucose 6–10 mmol L⁻¹ (108–180 mg dL⁻¹); Na⁺ 135–155 mmol L⁻¹.
Endocrine / Haemodynamic Optimisation
- Vasopressin 0.5–4 U h⁻¹ for vasoplegia and diabetes insipidus (DDAVP alternative).
- Corticosteroid: methylprednisolone 15 mg kg⁻¹ IV once (organ-protective, attenuates inflammation).
- Insulin infusion to target glucose above.
- Thyroid hormone (e.g., T₃ 4 µg IV bolus then 3 µg h⁻¹) consider if ventricular dysfunction and high vasoactive requirement persist—evidence is mixed; use selectively.
- Inotropy (dobutamine or milrinone) if low output after preload optimisation; minimise high-dose α-agonists.
- Re-image with transthoracic/ TOE after optimisation; stress, catecholamine-induced LV depression is often reversible.
Echocardiography and Acceptance
- Exclude major structural disease (LVH, significant valve lesions, congenital lesions).
- After optimisation, LVEF ≥45–50% and stable haemodynamics generally acceptable, considering recipient risk and centre thresholds.
- Note: DCD (donation after circulatory death) and normothermic ex-vivo perfusion are increasingly used to expand the donor pool; acceptance is protocoland centre-specific.
Physiology of the Transplanted (Denervated) Heart
- Denervation: no vagal/ sympathetic reflexes → resting HR typically 90–110 bpm; no response to atropine or carotid sinus massage; Frank–Starling mechanism intact; partial reinnervation can occur over years.
- Pharmacology: direct-acting agents work normally (adrenaline, noradrenaline, phenylephrine, isoprenaline). Ephedrine may be unreliable (indirect effect). Adenosine can cause exaggerated AV block/asystole—start with substantially reduced doses and have pacing ready.
- Arrhythmias/conduction: bradyarrhythmias and conduction defects are more common; pacemaker may be required.
- Ischaemia perception: angina may be absent; CAV often presents silently.
Key Responses (denervated heart)
| Stimulus | Response |
|---|---|
| Atropine / glycopyrrolate | No HR effect |
| Ephedrine | Reduced/unreliable |
| Direct catecholamines (adrenaline/noradrenaline) | Predictable effect |
| Carotid massage / Valsalva | No HR reduction |
| Pain / hypovolaemia | Delayed HR rise |
| Adenosine | Enhanced AV nodal block → use low dose |
Immunosuppression & Major Complications
Typical Regimens
- Induction (centre-specific): basiliximab or anti-thymocyte globulin in selected/high-risk recipients.
- Maintenance: tacrolimus + mycophenolate mofetil + prednisone (early triple therapy), with later minimisation when feasible; mTOR inhibitors (everolimus/sirolimus) for CAV or renal sparing (beware wound healing).
Toxicities
- Calcineurin inhibitors (tacrolimus/ciclosporin): nephrotoxicity, neurotoxicity (tremor, seizures), hypertension, hyperkalaemia, drug–drug interactions (macrolides/azoles ↑ levels).
- Mycophenolate: diarrhoea, leucopenia.
- Steroids: hyperglycaemia, infection, osteoporosis.
Infection
- Highest risk in first 6–12 months; institute TMP-SMX (PJP), CMV prophylaxis per serostatus, and antifungal prophylaxis as indicated.
Rejection
- Surveillance: scheduled endomyocardial biopsy (EMB) early post-transplant (ISHLT 0R–3R for cellular; pAMR for antibody-mediated), with gene-expression profiling and donor-derived cell-free DNA adjuncts in stable, low-risk patients to reduce biopsy burden.
- Incidence (modern era): treated rejection within year 1 occurs in roughly 15–25% of adults (centreand era-dependent).
- Treatment:
- ACR (≥2R): high-dose IV steroids ± ATG if refractory.
- AMR: combination of plasmapheresis, IVIG, rituximab; consider complement inhibitors and optimisation of maintenance therapy.
Chronic Allograft Vasculopathy (CAV)
- Diffuse, concentric coronary intimal thickening; often silent.
- Prevention/management: statins for all, aggressive risk-factor control, CMV prevention; consider mTOR-based regimens for progression.
- Surveillance: annual/periodic coronary angiography with IVUS/OCT (more sensitive for early disease); functional stress testing has lower sensitivity for microvascular disease.
Perioperative Management of Orthotopic Heart Transplantation (OHT)
Aims and Guiding Principles
- Safe induction and separation from cardiopulmonary bypass (CPB) with stable biventricular function, controlled pulmonary vascular resistance (PVR), and haemostasis.
- Early detection and treatment of primary graft dysfunction (PGD-Heart) and vasoplegic syndrome.
- Prompt initiation/continuation of immunosuppression, infection prophylaxis, and glycaemic control.
Pre-induction Checklist (recipient)
- Lines/monitoring: radial/femoral arterial line; large-bore central venous access (avoid chronic access sites); external defibrillator pads; transoesophageal echo (TEE) ready; pulmonary artery catheter (PAC) selectively (severe pulmonary hypertension, complex redo/LVAD explant).
- Airway/aspiration risk: advanced heart failure → delayed gastric emptying.
- Drug reconciliation: continue inotropes/vasodilators; hold ACEi/ARB morning of surgery if severe vasoplegia risk; confirm anticoagulation plan (heparin/warfarin/DOAC), antifibrinolytic strategy, and infection prophylaxis.
- Immunology/infection: confirm crossmatch/DSA status, CMV status, and induction plan.
- Bridged recipients (LVAD/VA-ECMO): anticipate adhesions, coagulopathy, RV vulnerability, and higher vasoplegia risk; plan blood products and MCS backup.
Induction & Maintenance
- Goals: preserve preload/afterload, avoid hypotension and acute ↑PVR (hypoxia/hypercarbia/acidosis/hypothermia/high PEEP).
- Induction options: fentanyl-based balanced anaesthesia with etomidate or ketamine (if shock); slow-titrated propofol acceptable in stable patients. Use cisatracurium or rocuronium (sugammadex available).
- Vasoactive readiness: start noradrenaline (e.g., 0.02–0.2 μg kg⁻¹ min⁻¹) early if vasoplegia-prone; add vasopressin (0.01–0.06 U min⁻¹) if catecholamine-resistant.
CPB Conduct (high-level)
- Heparinisation to target ACT per institutional protocol; antifibrinolytic (e.g., tranexamic acid 10–15 mg kg⁻¹ loading, then 1–2 mg kg⁻¹ h⁻¹).
- Ventilation on bypass: low Vt, low FiO₂; prevent atelectasis.
- Temperature/electrolytes: avoid hypothermia; correct Ca²⁺/K⁺/Mg²⁺.
Reperfusion and Separation from CPB
- TEE-guided assessment: biventricular systolic/diastolic function, volume status, valve competence, anastomoses, air, outflow kinks, pericardial collections.
- Inotrope/vasopressor strategy (typical ranges):
- Adrenaline 0.02–0.10 μg kg⁻¹ min⁻¹ ± dobutamine 2–10 μg kg⁻¹ min⁻¹ or milrinone 0.25–0.5 μg kg⁻¹ min⁻¹ (avoid bolus).
- Noradrenaline 0.02–0.2 μg kg⁻¹ min⁻¹ for MAP; vasopressin 0.01–0.06 U min⁻¹ for vasoplegia.
- Inhaled pulmonary vasodilators for RV afterload: nitric oxide 10–40 ppm or inhaled epoprostenol 20–50 ng kg⁻¹ min⁻¹.
- Pacings wires: establish AV synchrony; treat junctional rhythm/bradyarrhythmia promptly.
Primary Graft Dysfunction (PGD—Heart)
- Definition: new LV/RV/biventricular failure within 24 h of reperfusion, not due to hyperacute rejection or surgical cause; graded by severity and need for mechanical circulatory support (MCS). PGD is the leading cause of early mortality.
- Risk factors: prolonged ischaemic time, marginal/DCD donors, LVAD bridge, severe pulmonary hypertension, transfusion/CPB injury.
- Management: optimise preload/afterload; high-dose inotropes only as bridge; early consideration of VA-ECMO if failure to separate from CPB or escalating support—do not delay rescue MCS. Exclude technical issues (kink/tamponade), AMR, and severe vasoplegia.
Vasoplegic Syndrome after CPB (including during OHT)
- Phenotype: hypotension with low SVR and normal/high CO, catecholamine-resistant.
- First-line: noradrenaline ± vasopressin; correct acidosis/hypocalcaemia/hypothermia.
- Rescue therapies (centre-specific):
- Methylene blue 1–2 mg kg⁻¹ IV over 20–60 min (avoid with serotonergics/MAOIs; caution G6PD deficiency).
- Hydroxocobalamin 5 g IV over 10–15 min (may repeat once) as alternative/adjunct.
- Angiotensin II infusion where available.
Haemostasis and Transfusion
- Protamine titration to reverse heparin; viscoelastic-guided products.
- Targets commonly used: Hb 8–10 g dL⁻¹, platelets >100 ×10⁹ L⁻¹, fibrinogen >1.5–2.0 g L⁻¹; minimise allogeneic exposure.
- Re-exploration threshold low if ongoing bleeding with tamponade risk
Early Post-operative Management (ICU)
- Ventilation: lung-protective; avoid excessive PEEP (RV preload dependence); aim normocapnia and adequate oxygenation.
- Glycaemic control: IV insulin to 6–10.0 mmol L⁻¹
- Immunosuppression: per protocol—high-dose methylprednisolone intra-op; start tacrolimus (timed to renal function) + mycophenolate + prednisone; ATG/basiliximab for induction in selected patients. Infection prophylaxis (CMV, PJP) per serostatus.
- Surveillance: daily echo as indicated; early ECG/enzymes; consider EMB schedule; strict renal monitoring and VTE prophylaxis.
Special Situations
- Donation after circulatory death (DCD) hearts: higher PGD risk; centres use normothermic ex-vivo perfusion or thoraco-abdominal normothermic regional perfusion (NRP) protocols; anticipate longer ischaemic times and lower threshold for early VA-ECMO.
- LVAD explant/re-transplant: anticipate dense adhesions, coagulopathy, RV failure; ensure additional blood products, meticulous haemostasis, and lower threshold for MCS.
Fast Reference (doses & targets)
- Vasopressin: 0.01–0.06 U min⁻¹.
- Noradrenaline: 0.02–0.2 μg kg⁻¹ min⁻¹.
- Adrenaline: 0.02–0.10 μg kg⁻¹ min⁻¹.
- Dobutamine: 2–10 μg kg⁻¹ min⁻¹.
- Milrinone: 0.25–0.5 μg kg⁻¹ min⁻¹ (avoid bolus).
- iNO: 10–40 ppm; inhaled epoprostenol: 20–50 ng kg⁻¹ min⁻¹.
- Methylene blue: 1–2 mg kg⁻¹ IV; Hydroxocobalamin: 5 g IV.
Anaesthesia for Cardiac Transplant Recipients
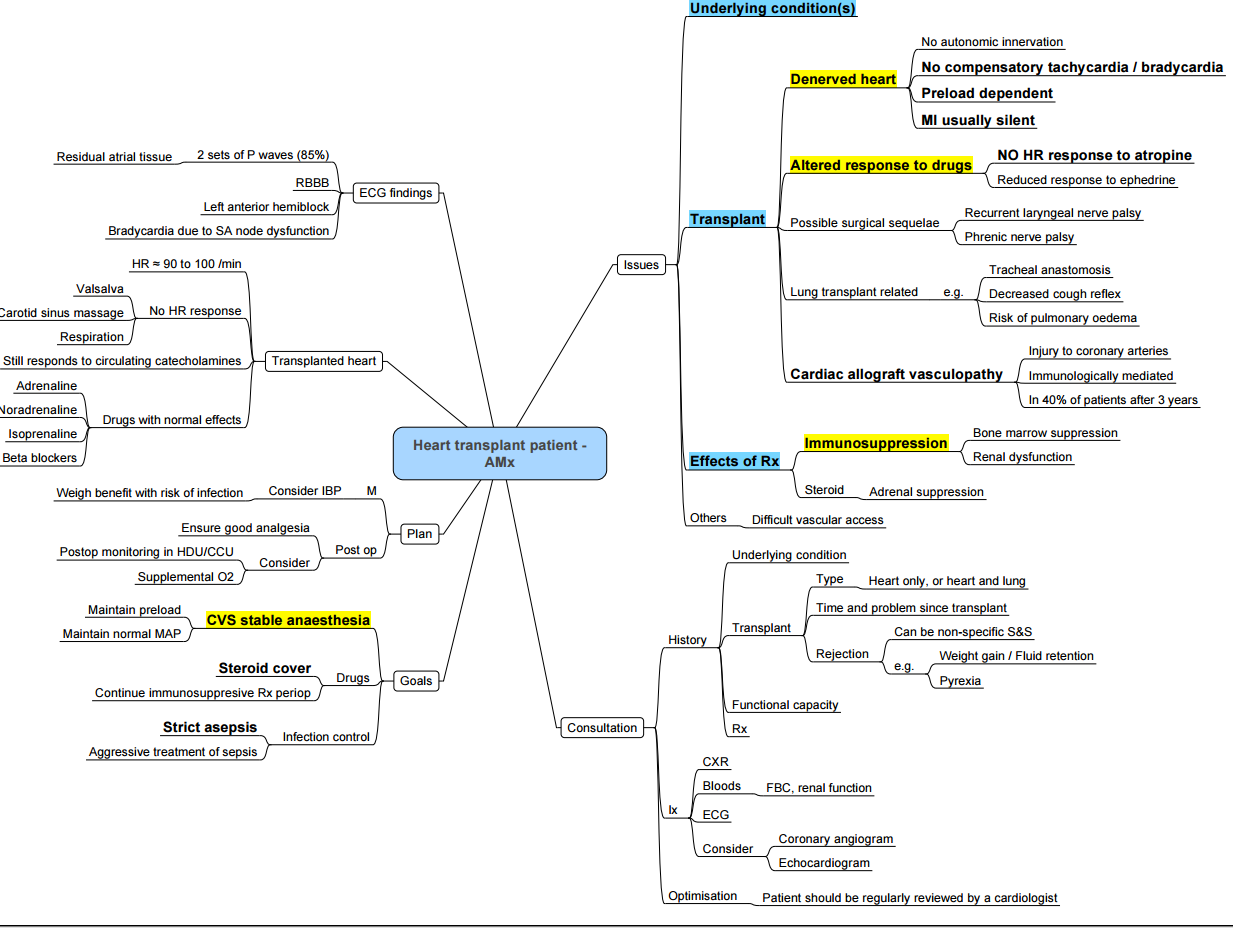
Anaesthetic Concerns in Recipients of Heart Transplants Presenting for Non-Transplant Surgery
View or edit this diagram in Whimsical.
Lung Transplant
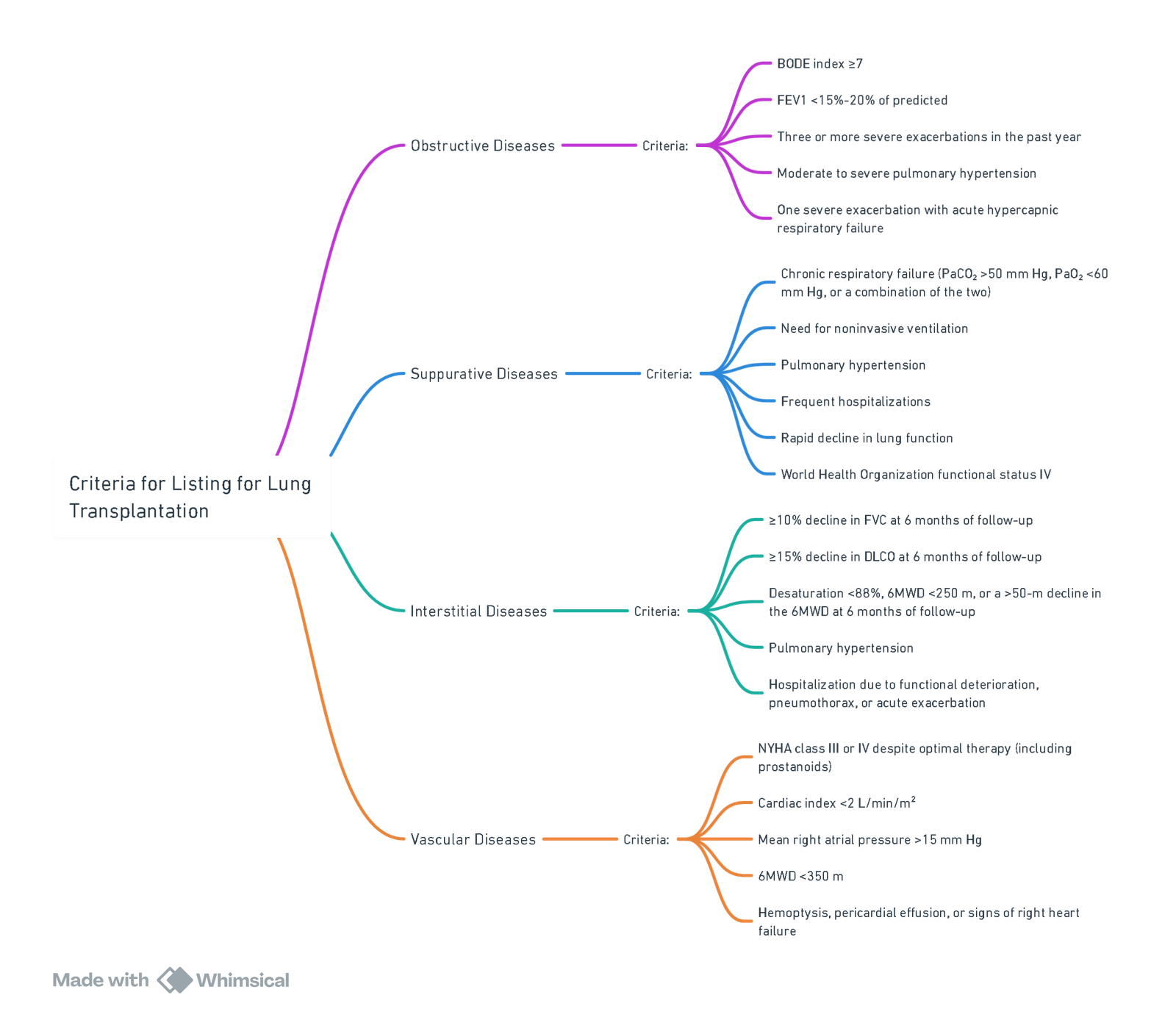
Criteria for Listing for Lung Transplantation for Different Underlying Lung Pathologies
View or edit this diagram in Whimsical.
Criteria for Listing for Lung Transplantation (by Underlying pathology)
- Principle. List when the expected survival benefit outweighs risk: typically >50% two-year mortality without transplant and >80% likelihood of short-term survival with transplant. Timing is earlier for rapidly progressive diseases (e.g., fibrotic ILD).
Chronic Obstructive Pulmonary Disease (COPD)
- Strong indicators for listing (any one, after optimisation and MDT review):
- BODE score ≥7 or rapid BODE rise.
- FEV₁ <20% predicted with either DLCO <20% or severe hyperinflation, and/or pulmonary hypertension or chronic hypercapnia.
- Frequent severe exacerbations (e.g., ≥3/year needing IV therapy or ≥1 ICU/NIV episode) or rapid decline despite maximal therapy and pulmonary rehabilitation.
Interstitial Lung Disease (ILD) including Idiopathic Pulmonary Fibrosis (IPF)
- List if progressive disease despite appropriate therapy, typically when any of:
- Absolute FVC decline >10% in ≤6 months; DLCO decline >10–15% in ≤6 months; or FVC decline >5% plus radiographic progression.
- Rest or exertional desaturation <88% (6-minute walk test) or >50 m fall in 6MWT over 6 months.
- Pulmonary hypertension on echo/RHC, recurrent hospitalisation, AE-ILD, or pneumothorax. Continue antifibrotics (pirfenidone, nintedanib) to transplant.
Cystic Fibrosis (CF) and Bronchiectasis (suppurative disease)
- List if one or more of:
- FEV₁ <30% predicted (adults) or <40% (children), or FEV₁ <50% with rapid decline.
- 6MWT <400 m, PaCO₂ >50 mmHg, hypoxaemia, pulmonary hypertension, ≥2 IV-treated exacerbations/year, massive haemoptysis needing embolisation, or pneumothorax.
- Colonisation/infection with high-risk organisms (e.g., Burkholderia cepacia complex—especially B. cenocepacia—or Mycobacterium abscessus): not universally absolute but requires centre expertise and rigorous protocols.
Pulmonary Arterial Hypertension (PAH, WHO Group 1)
- List if high-risk features persist despite optimal therapy (including parenteral prostacyclin where indicated), e.g.:
- NYHA/WHO III–IV, REVEAL 2.0 high risk, right-atrial pressure >15 mmHg, cardiac index <2.0 L min⁻¹ m⁻², 6MWD <350 m, syncope, progressive RV failure, or pericardial effusion. Consider bilateral lung vs heart–lung on centre criteria.
Contraindications to Lung Transplantation
Absolute (typical)
- Active malignancy with high risk of recurrence (disease-free interval requirements depend on histology and risk; many solid tumours require ≥2–5 years disease-free)
- Untreatable, significant dysfunction of another major organ (unless multi-organ transplant planned).
- Uncontrolled acute medical instability (e.g., sepsis, shock, AMI, acute liver failure).
- Active substance use (nicotine—including vaping—alcohol, illicit drugs); documented non-adherence after remediation efforts.
- Active, uncontrolled infection that is untreatable or carries prohibitive risk (e.g., disseminated moulds such as Lomentospora).
- Body mass index (BMI) ≥35 kg m⁻² (strong adverse outcome signal).
- Absence of social support or progressive cognitive/psychiatric disease precluding adherence.
- Active, untreated tuberculosis (TB). Latent TB is not an absolute contraindication but requires management per transplant ID protocols.
Relative (context-dependent; centre-specific)
- Older age: no fixed upper limit, but outcomes decline with age; >70–75 years seldom candidates unless exceptional physiology/support.
- BMI 30–34.9 kg m⁻² or frailty, hypoalbuminaemia, and severe osteoporosis—optimise before listing.
- Colonisation with highly drug-resistant organisms (e.g., B. cenocepacia, NTM): high risk but not universally preclusive at experiened centres.
- Atherosclerotic vascular disease with limited reserve; CAD acceptable if adequately revascularised.
- HIV, HBV, HCV: acceptable if well-controlled on therapy with expert management.
Conduct of Anaesthesia for Lung Transplant
Pre-operative Assessment (what Changes Your plan)
| Assessment | Anaesthetic/Peri-op implications |
|---|---|
| Underlying diagnosis & physiology (obstructive/restrictive/PAH) | Ventilator mode and permissive targets; anticipate OLV tolerance, air-trapping, PVR triggers |
| Pulmonary function tests (spirometry, DLCO) | Predict OLV/ventilation strategy; risk of dynamic hyperinflation vs low compliance |
| ABG and 6MWT/CPET | Set acceptable intra-op limits; anticipate hypercapnia or hypoxaemia tolerance |
| V/Q or quantitative CT | Guides sequence (which lung first) and tolerance of clamping/OLV |
| PAP/RHC, echo (RV function) | Need for inotropes/vasodilators; threshold for ECLS (ECMO/CPB); cannulation plan |
| Sensitisation (DSA/HLA), crossmatch | Blood product and immunosuppression strategy; risk of PGD/AMR |
- Medication continuity: Continue bronchodilators, inhaled steroids, pulmonary vasodilators, CF airway regimens and antibiotics. Pre-op broad-spectrum and antifungal therapy per microbiology.
- Analgesia planning: Thoracic epidural analgesia (TEA) can provide excellent pain control but is often impractical due to urgent timing and peri-operative anticoagulation (ECMO/CPB). Consider paravertebral or erector spinae plane blocks and multimodal analgesia.
Intra-operative Management
- Monitoring and imaging
- Invasive arterial line, large-bore central venous access, near-patient ABGs.
- Transoesophageal echo (TOE): assess RV size/function, guide volume/inotrope strategy, detect air/thrombus, evaluate anastomotic flow after implantation.
- Pulmonary artery catheter as per centre practice and RV/PAH severity.
- Airway & ventilation
- DLT or SLT with blocker as appropriate; ensure meticulous bronchoscopic toileting and confirmation of anastomoses.
- During OLV (recipient): Vₜ 4–6 mL kg⁻¹ IBW, titrate PEEP 3–10 cm H₂O to best compliance; plateau <30 cm H₂O or driving pressure <14 cm H₂O; permissive hypercapnia if haemodynamically tolerated.
- Ventilation of the transplanted lung(s)
- Lung-protective ventilation, indexed to the donor predicted body weight (dPBW) for double-lung grafts: Vₜ 6–8 mL kg⁻¹ dPBW, plateau <30 cm H₂O, moderate PEEP; avoid overdistension.
- Lowest FiO₂ that maintains PaO₂ ≥ 70 mmHg; careful stepwise recruitment; bronchoscopic clearance early.
- Haemodynamics
- Goals: preserve RV perfusion and output; avoid ↑PVR (hypoxia, hypercarbia, acidosis, hypothermia, high PEEP, high airway pressures, pain).
- Vasoactive support as needed (e.g., noradrenaline, vasopressin for SVR; adrenaline/milrinone/dobutamine for RV; inhaled nitric oxide or epoprostenol for acute ↑PVR).
- Fluids: restrictive, balanced crystalloids; avoid excessive transfusion (see PGD).
- Extracorporeal support (ECLS)
- Off-pump preferred when feasible. If support needed, VA-ECMO is generally favoured over CPB (less inflammatory activation, bleeding, transfusion, and lower PGD risk than CPB). Observational registries show PGD risk: off-pump < ECMO < CPB.
- Donor ventilatory care (for retrieval teams)
- Protective ventilation: Vₜ 6–8 mL kg⁻¹, PEEP 8–10 cm H₂O, regular recruitment maneuvers, avoid derecruitment during apnea testing (use CPAP). This strategy increases utilisation and may improve recipient outcomes.
- Immunosuppression (peri-op)
- Protocols vary; a common approach is induction with basiliximab (e.g., 20 mg on day 0 ± day 4) or ATG, plus high-dose corticosteroid (e.g., methylprednisolone 500–1,000 mg IV at implantation/reperfusion), then maintenance with tacrolimus, mycophenolate mofetil, and prednisone. Early tacrolimus is usual; targets and timing are centre-specific (consider renal function).
Fluid Therapy during Lung Transplantation
Overarching Principles
- Vasopressor-first, fluid-sparing. Treat anaesthetic vasodilation and hypotension primarily with noradrenaline (± vasopressin) and add inotropes for RV support; give small, diagnostic boluses (100–250 mL) of balanced crystalloid only when there is echo/clinical evidence of underfilling. Liberal fluid loads increase PGD risk.
- Use the right monitors. CVP/PPV/SVV are unreliable with open chest/OLV; base decisions on TEE (RV size/function, LV filling), gas exchange, lactate, and (when used) PAC trends.
- Choose fluids wisely. Prefer balanced crystalloids (e.g., Plasma-Lyte/LR) to avoid hyperchloraemic acidosis (↑PVR, AKI signal). Avoid hydroxyethyl starch (HES) because of AKI/coagulopathy warnings. Use 5% albumin selectively when oncotic support is needed and large crystalloid volumes would otherwise be required.
Phase-specific Targets
1) Pre-reperfusion (dissection, First Implantation, OLV)
- Aim MAP ≥65 mmHg, CI >2.2 L min⁻¹ m⁻² (if measured), SpO₂ ≥92–94%; avoid PVR triggers (hypoxia, hypercarbia, acidosis, hypothermia, high PEEP/plateau).
- Give small crystalloid boluses only if TEE/PAC suggests preload dependence; otherwise escalate noradrenaline 0.02–0.2 µg kg⁻¹ min⁻¹ ± vasopressin 0.01–0.06 U min⁻¹ and add inodilator for RV (dobutamine/milrinone).
2) Immediate Post-reperfusion (each lung)
- Keep left-sided filling pressures low to limit reperfusion oedema: target low wedge/left-atrial pressure (pragmatically ≈5–15 mmHg) while maintaining perfusion (MAP ≥65 mmHg, UO ≥0.5–1 mL kg⁻¹ h⁻¹). Prioritise vasopressors over fluid for hypotension unless clear hypovolaemia. Consider early diuretics if left-sided pressures or O₂/complaince worsen.
3) If on Intra-op ECMO/CPB
- Avoid excessive volume to “chase” flows; use vasopressors and ventilator adjustments. ECMO allows net-even to negative balance once stable; de-air/TEE checks before weaning. ECMO generally carries less PGD risk than CPB, but off-pump < ECMO < CPB overall.
How much Fluid?
- There is no fixed litre target; the goal is euvolaemia with the smallest positive balance compatible with perfusion. Observational data associate greater intra-op fluid volumes with higher grade-3 PGD—treat fluids like a drug and titrate in 100–250 mL steps to objective endpoints. Many centres aim to avoid >1–2 L net positive when bleeding is modest.
Blood Products (patient Blood management)
- Restrictive RBC strategy (contextualised to O₂ delivery/RV load): general guidance supports Hb ~70–80 g L⁻¹ as a trigger unless there is active ischaemia, severe hypoxaemia, or profound RV strain. >4 units PRBC intra-op associates with higher grade-3 PGD—avoid unless essential. Use leucocyte-reduced components.
- Plasma/platelets/fibrinogen: viscoelastic-guided replacement; avoid high FFP:RBC ratios unless bleeding/coagulopathy dictates (linked to worse outcomes in some series). Suggested targets: platelets >100 ×10⁹ L⁻¹, fibrinogen >1.5–2.0 g L⁻¹.
- Cell salvage is reasonable; rinse thoroughly to reduce cytokines. (Centre-specific.)
Practical Bedside Algorithm (recipient)
- Hypotension?
- Echo underfilled? Give 100–250 mL balanced crystalloid, reassess.
- Not underfilled? Start/ups titrate noradrenaline; add vasopressin if catecholamine-resistant; add inodilator for RV dysfunction.
- After reperfusion: keep wedge/LA pressure low (≈5–15 mmHg); diurese early if compliance/O₂ drop with rising pressures; avoid fluid boluses unless clear hypovolaemia.
- Bleeding/coagulopathy: run goal-directed PBM with viscoelastic testing; keep RBCs restrictive; avoid HES.
- Escalate to VA-ECMO early for evolving PGD with hypoxaemia/RV failure, rather than escalating fluids/inotropes.
Challenges and Strategies by Recipient Pathology
| Pathology | Typical intra-op issues | Practical strategies |
|---|---|---|
| Obstructive (COPD, BOS) | Dynamic hyperinflation, auto-PEEP, air-trapping | Pressure-controlled or low Vₜ; I:E 1:3–1:4; minimal external PEEP (≈3–4 cm H₂O); allow prolonged exhalation; check for expiratory flow-limitation |
| Suppurative (CF, bronchiectasis) | Thick/purulent secretions; high airway resistance | Initial SLT for BAL/suction then DLT; higher PEEP (8–10) as needed; frequent bronchoscopic toileting; expect hypercapnia |
| Restrictive (fibrosis, HP) | Poor compliance; pulmonary hypertension | Short inspiratory holds; I:E 1:1–1:2; cautious higher PEEP (8–10) if recruitable; anticipate ECMO threshold |
| Severe PAH | RV failure, hypotension with induction/OLV | Central access before induction; inodilators/vasopressors ready; continue prostanoids; inhaled NO/epoprostenol; low threshold for VA-ECMO |
- If induction not tolerated: consider awake/ECMO-assisted strategy, PA clamping tests, early OLV, or transition to CPB/ECMO.
Cardiopulmonary Bypass (CPB) Vs ECMO — Practical pros/cons
CPB
- Pros: familiar; easy volume addition; facilitates combined cardiac surgery.
- Cons: more haemodilution/inflammation, higher bleeding and transfusion, higher PGD than ECMO; often longer ventilation/ICU.
ECMO (usually VA)
- Pros: lower anticoagulation dose than CPB; less bleeding/transfusion; facilitates lung-protective ventilation; useful as bridge and for immediate post-op support.
- Cons: If prolonged bridge (>~2 weeks) or severe pre-op deconditioning, survival declines; still greater PGD risk than off-pump.
Primary Graft Dysfunction (PGD)
- Definition/grading: acute lung injury within 72 h post-implant; graded 0–3 by PaO₂/FiO₂ and CXR infiltrates (ISHLT system). PGD is the leading cause of early morbidity/mortality.
- Risk factors (operative): CPB use; high transfusion burden (e.g., >4 units PRBCs intra-op); donor/recipient factors. Minimise allogeneic exposure and avoid liberal FFP.
- Management: lung-protective ventilation, high PEEP/FiO₂ as needed, inhaled vasodilators, diuresis/ultrafiltration, and VA-ECMO for refractory hypoxaemia/RV failure. Exclude technical problems (e.g., pulmonary vein thrombosis).
Anaesthetic Concerns in Recipients Presenting for Non-transplant Surgery
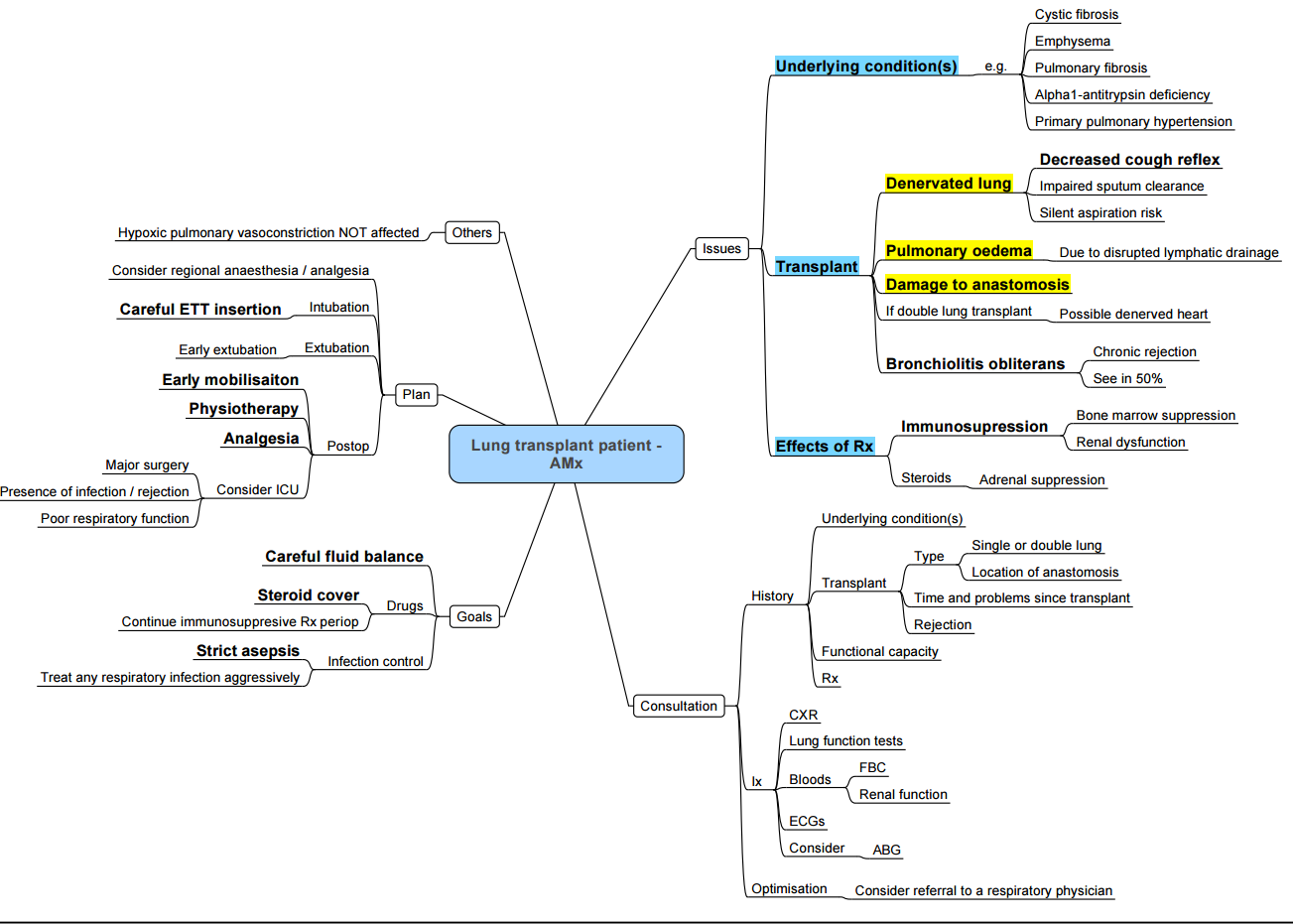
- Allograft physiology: heterogeneous compliance, impaired cough, disrupted lymphatics → infection risk, secretion retention.
- Single-lung recipients: consider differential ventilation; avoid overdistending the graft; CPAP to native lung if severe V/Q mismatch.
- Pulmonary hypertension and RV function: remain vigilant for PVR triggers; have inhaled vasodilators available.
- Immunosuppression: strict asepsis; avoid nephrotoxins (calcineurin inhibitors); recognise haematologic effects (anaemia, leucopenia).
- Airway & anastomoses: minimise airway trauma; consider bronchoscopy if concerns about stenosis/anastomotic integrity.
- Peri-op strategy: regional/neuraxial when feasible and safe; restrictive fluids; antimicrobial prophylaxis tailored to colonisation history.
Links
- Cardiac physiology
- Respiratory physiology and Thoracic anaesthesia
- Transplants and organ donation
- Liver transplant
- Thoracic pre-op assessment
References:
- Pařízková, B. and Wright, I. G. (2015). Cardiopulmonary transplantation. Anaesthesia &Amp; Intensive Care Medicine, 16(10), 513-516. https://doi.org/10.1016/j.mpaic.2015.07.002
- Peled Y, et al. ISHLT Guidelines for the evaluation and care of cardiac transplant candidates. J Heart Lung Transplant. 2024. Available at ISHLT. DefaultJHLT Online
- Velleca A, et al. ISHLT Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2023;42(5):e1–e140. (PDF available via ISHLT). PubMedDefault
- Copeland H, et al. Donor heart selection: evidence-based guidelines for providers. J Heart Lung Transplant. 2022–2023. (Open-access version). PMCJHLT Online
- Seshadri A, et al. Organ donation in the surgical ICU: an American perspective. Trauma Surg Acute Care Open. 2023;8:e001107. (Best-practice donor optimisation overview). TSACO
- Donor Management Goals (DMG) Registry resources and outcomes. National DMG. Accessed 2024–2025. Donor Management Goals+1
- Khush KK, et al. Dual non-invasive surveillance and acute rejection incidence in the first year. J Heart Lung Transplant. 2024. JHLT Online
- Gemelli M, et al. Rejection requiring treatment within the first year after heart transplantation: UNOS 2000–2021. J Clin Med. 2023;12(23):. PMC
- Flyer JN, et al. Adenosine effects in heart transplant recipients—dose implications. Circulation. 2017;135:1828–1837. (Open-access summary). PMC
- Leard LE, Holm AM, Valapour M, et al. Consensus document for the selection of lung transplant candidates: An update from the ISHLT. J Heart Lung Transplant. 2021;40(11):1349-1379. Available via PMC: https://pmc.ncbi.nlm.nih.gov/articles/PMC8979471/
- Will D. Lung transplantation: indications and contraindications. J Thorac Dis. 2018;10(7):4574-87. https://pmc.ncbi.nlm.nih.gov/articles/PMC6105990/
- Snell GI, Yusen RD, Will D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction: Part I—Definition and grading. J Heart Lung Transplant. 2017;36(10):1097-1103.
- Mascia L, Pasero D, Slutsky AS, et al. Effect of a lung-protective strategy for organ donors on eligibility and utilisation. JAMA. 2010;304(23):2620-7.
- Biscotti M, Yang J, Sonett J, Bacchetta M. Comparison of ECMO vs CPB during lung transplantation. J Thorac Cardiovasc Surg. 2014;148(5):2410-2415.
- Loor G, Sharma S, et al. Effect of mode of intraoperative support on severe PGD: international registry analysis. J Thorac Cardiovasc Surg. 2022;163(5):1750-1761.
- Magouliotis DE, et al. ECMO vs CPB in lung transplantation: meta-analysis. Interact Cardiovasc Thorac Surg. 2018;26(2):312-321.
- Subramaniam K, et al. Intraoperative PRBC transfusion >4 units and PGD risk. Anesth Analg. 2023;136(4):773-783.
- Seay TD, et al. FFP:RBC ratio and PGD in bleeding LT patients. J Cardiothorac Vasc Anesth. 2020;34(11):2994-3003.
- Nesseler N, et al. Perioperative management of heart transplantation: clinical review. Anesthesiology. 2023;139:493–510. PubMed
- Edwards S, Allen S, Sidebotham D. Anaesthesia for heart transplantation. BJA Educ. 2021 Aug;21(8):284-291. doi: 10.1016/j.bjae.2021.02.006. Epub 2021 Apr 27. PMID: 34306729; PMCID: PMC8283718.
- Durkin, C. and Buckland, M. (2015). Cardiopulmonary transplantation: anaesthetic implications. Anaesthesia &Amp; Intensive Care Medicine, 16(7), 324-327. https://doi.org/10.1016/j.mpaic.2015.04.010
- Choudhury M. Post-cardiac transplant recipient: Implications for anaesthesia. Indian J Anaesth. 2017 Sep;61(9):768-774. doi: 10.4103/ija.IJA_390_17. PMID: 28970636; PMCID: PMC5613603.
Summaries:
Lung transplant anaesthesia- video
Post op considerations for lung transplant-video
Copyright
© 2025 Francois Uys. All Rights Reserved
id: “4e7e233d-d01f-4716-b12f-d20d868b7b07”



