- Summary
- Ischemic Heart Disease (IHD)
- Conduct of Anaesthesia for IHD in Non Cardiac Surgery
- Peri-Operative Myocardial Infarction (MI)
- Stents
- Links
{}
Summary
Ischemic Heart Disease (IHD)
Pathogenesis of Various Types of IHD
Factors Contributing to Atherosclerosis
- High Serum LDL: ↑ Availability of lipids that deposit in arterial wall.
- Low Serum HDL: ↓ Removal of LDL from coronary artery walls (transport of LDL to liver is impaired).
- Endothelial Dysfunction: Compromise of endothelial barrier → vessel wall vulnerable to infiltration by LDL and cells of the immune system.
Atherosclerosis
- Arterial wall degeneration, characterized by fat deposition in and fibrosis of the inner layer of arteries.
- Occurs in Coronary Arteries:
Stable Angina
- Stable Atheromatous Plaque:
- Fibromuscular cap overlying fatty plaque contents remains intact, and plaque contents are not released into the vessel lumen.
- Plaque serves as a fixed luminal obstruction to blood flow.
- If vessel stenosis is significant (≥70%), myocardial oxygen demand starts to exceed supply, especially with exertion.
- Predictable, transient myocardial ischemia.
Unstable Angina
- Unstable Atheromatous Plaque:
- Fibromuscular cap overlying fatty plaque ruptures.
- Thrombogenic plaque contents (especially tissue factor) are exposed to coagulation factors in the vessel lumen.
- Activation of platelets and the clotting cascade at the site of rupture.
- Thrombus forms over already partially occlusive plaque → occludes lumen → ↓ perfusion of myocardium.
- Transient ischemia of cardiomyocytes.
Myocardial Infarction (MI)
- Infarction (death) of cardiomyocytes.
Acute Coronary Syndromes (ACS)
- Includes Unstable Angina and Myocardial Infarction.
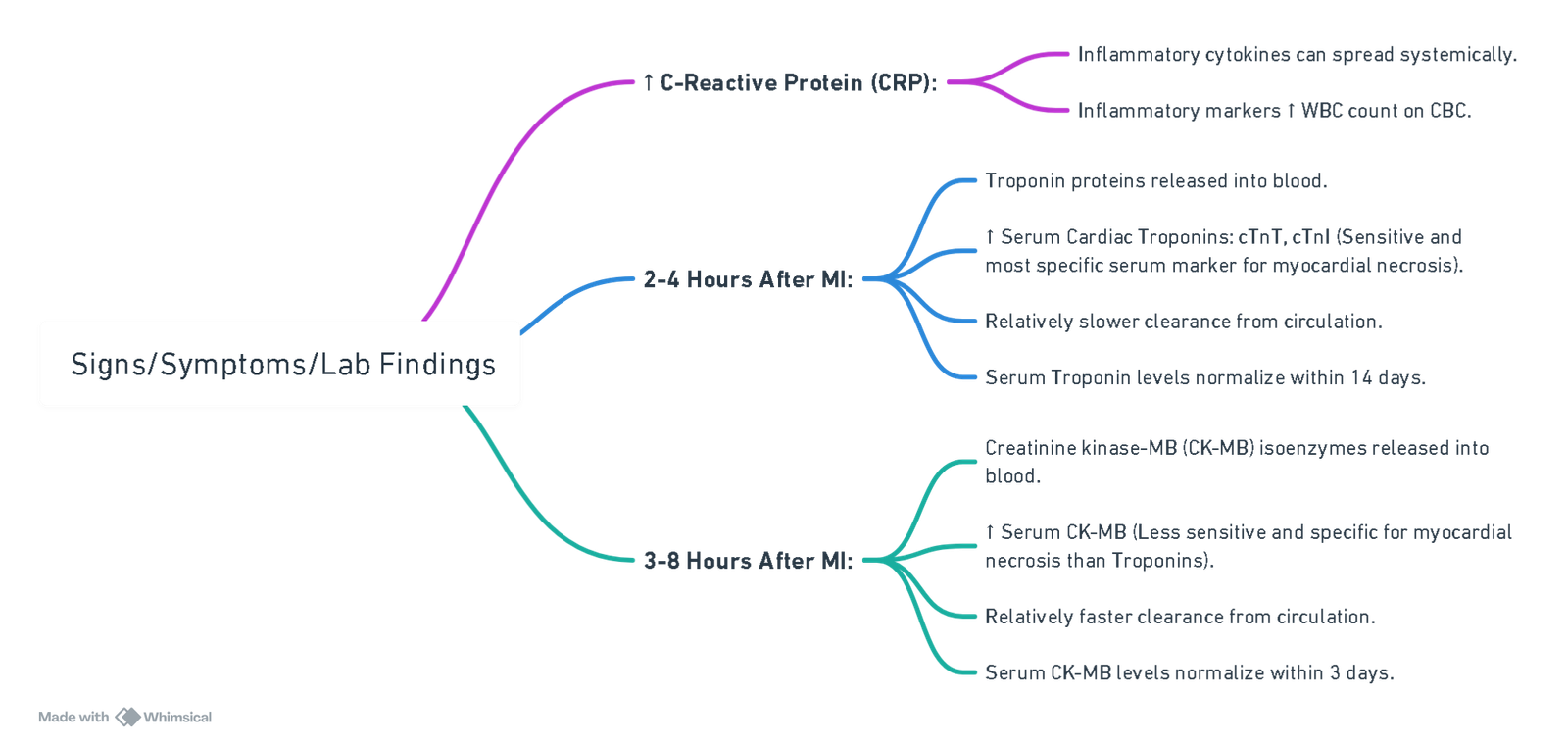
Myocardial Infarction: Findings on Investigations
View or edit this diagram in Whimsical.
Complications
- Tissue Ischemia: Disrupts normal cardiac electrical conduction (detected on serial ECG).
- ST-Segment Depression: Non-localizing, ischemia of sub-endocardial myocardium.
- ST-Segment Elevation: Localizes to site of ischemia, acute, trans-mural myocardial ischemia.
- If ischemia progresses to tissue infarction, Pathologic Q-waves form (localizes to site of ischemia).
Additional Notes
- Both types of ST-segment changes can indicate myocardial infarctions but can also be false positives (e.g., caused by left ventricular hypertrophy, bundle branch blocks, and other non-myocardial ischemic causes).
Complications of MI
Pathophysiology
Cardiac Contractility Due to Death of Cardiomyocytes
- Inadequate Cardiac Output:
- Stasis of blood in the ventricle.
- Formation of mural thrombus on akinetic inflamed wall segments.
- Embolization of thrombus.
- Systemic emboli (i.e., stroke, renal infarction, limb infarction).
Necrosis of Ventricular Wall 2° to Infarction
- Transmural Necrosis:
- Dead tissue disrupts cardiac conduction system (e.g., bundle of His).
- Causes acquired ventricular septal defect (VSD) and acquired mitral regurgitation (MR).
Irritability by Viable Tissue Adjacent to Area of Infarct
- Myocardial Electrical Instability:
- Ectopic beats, re-entry circuits.
- Necrotic tissue irritates and inflames pericarditis.
Complications
Pump Dysfunction
- Profound/Acute Dysfunction:
- Cardiac output ↓↓↓.
- Cardiogenic shock: ↓ Systemic & myocardial perfusion.
- Acidemia, systemic organ failure (renal failure, myocardial damage).
- Less Profound/Acute Dysfunction:
- Congestive heart failure.
- Ventricular aneurysm: Atria distend due to stasis, disrupts electrical conduction, atrial fibrillation.
Ventricular Free Wall Rupture
- If pericardial adhesion is covering the rupture, blood from rupture is contained within the adhesion.
- False aneurysm: If adhesion bursts.
- Cardiac tamponade: If excess inflammatory fluid/edema accumulates within the pericardial sac.
Arrhythmias
- Myocardial electrical instability, ectopic beats, re-entry circuits
Pericarditis
- Inflammatory process.
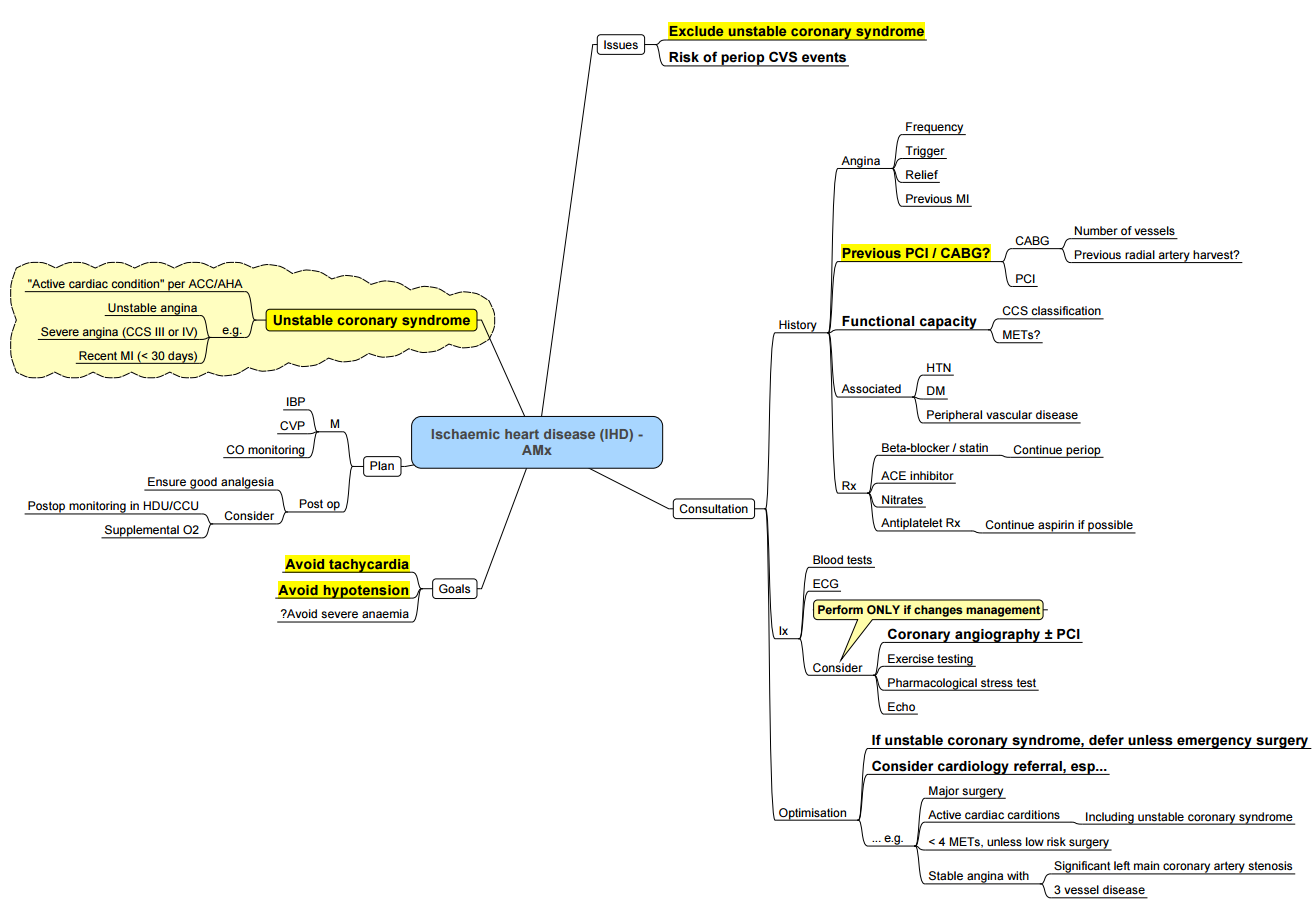
Conduct of Anaesthesia for IHD in Non Cardiac Surgery
Goals
View or edit this diagram in Whimsical.
Pre-Operatively
Goals of Pre-Op Assessment
- Current medical status
- Provide clinical risk profiling
- Decide on further testing
- Treat modifiable risk factors
- Plan management of cardiac illness during the peri-operative period
History and Optimization
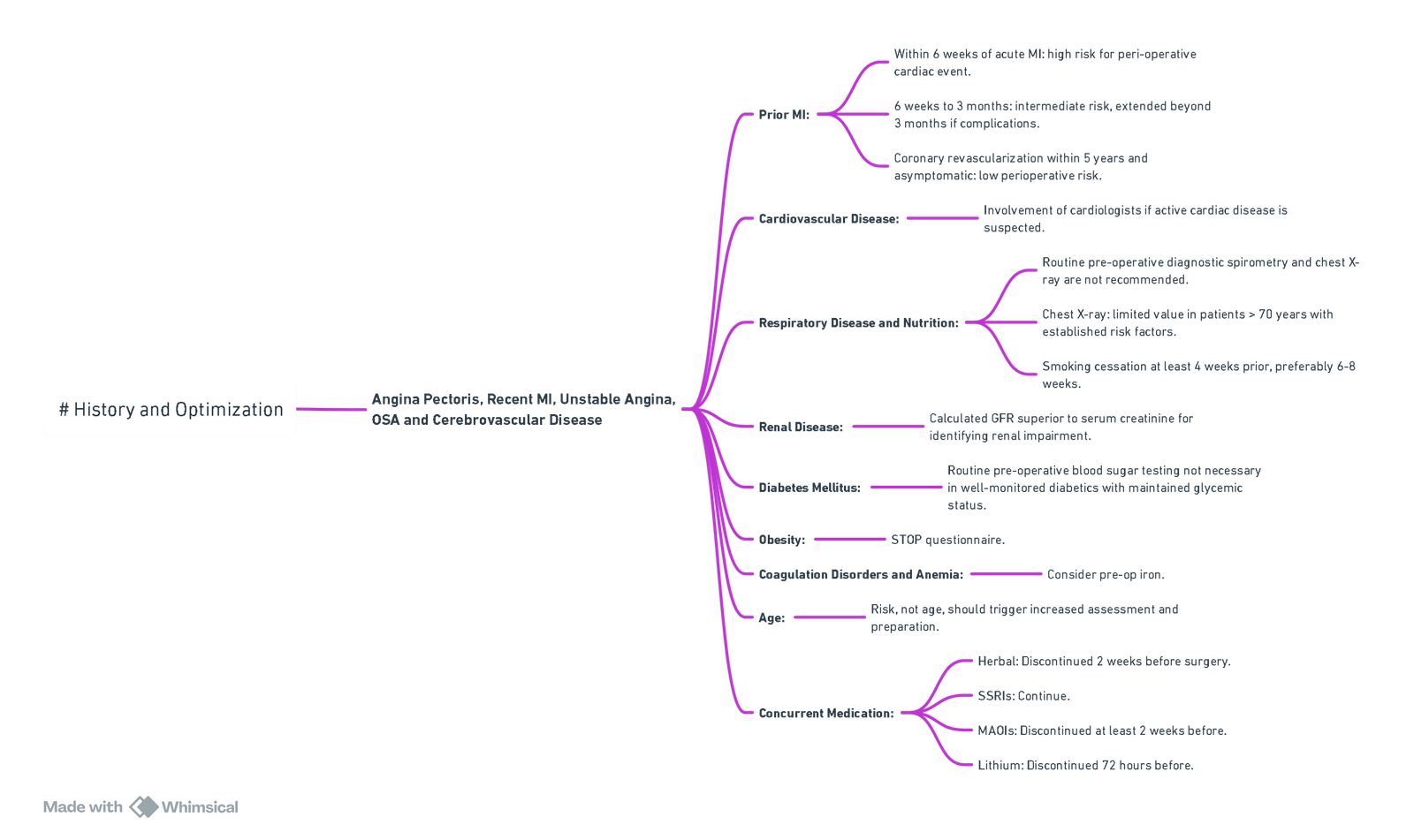
View or edit this diagram in Whimsical.
Pre-Op Testing
- ECG:
- Detects myocardial ischemia, MI, cardiac rhythm/conduction disturbances, ventricular hypertrophy, and electrolyte abnormalities.
- Peri-Operative Coronary Angiography:
- High risk of adverse outcome based on non-invasive test results.
- Angina pectoris unresponsive to medical therapy.
- Unstable angina, particularly with intermediate or high-risk non-cardiac surgery.
- Equivocal non-invasive test results in high-risk patients undergoing high-risk surgery.
Susceptibility to Perioperative Ischemia
- Patient Factors: Co-morbidities (e.g., cerebrovascular disease, AF, elderly, PFO, IE, DM, male, smoker).
- Surgical Factors: High-risk surgery (vascular, neuro, cardiac).
Intraoperative Adverse Events:
- Arrhythmias, hypotension, CPR, embolic phenomenon.
Risk Stratification
- Type of surgery, presence/type of clinical indicators of coronary artery disease, and patient functional status.
- Low-Risk Procedure: Combined surgical and patient characteristics predict a MACE risk of death or MI of <1%. Procedures with a risk of MACE of ≥1% are considered elevated risk.
Clinical Predictors of Increased Peri-Operative Cardiovascular Risk
- Level of Risk: Major (Cardiac Risk > 5%)
- Unstable coronary syndromes
- Decompensated CHF
- Significant arrhythmias
- Severe valvular disease
- Level of Risk: Intermediate (Cardiac Risk < 5%)
- Mild angina pectoris
- Prior MI
- Compensated/prior HF
- Diabetes mellitus (particularly on insulin)
- Renal insufficiency
- Level of Risk: Minor (Cardiac Risk < 1%)
- Advanced age
- Abnormal ECG
- Rhythm other than sinus
- Low functional capacity
- History of stroke
- Uncontrolled systemic hypertension
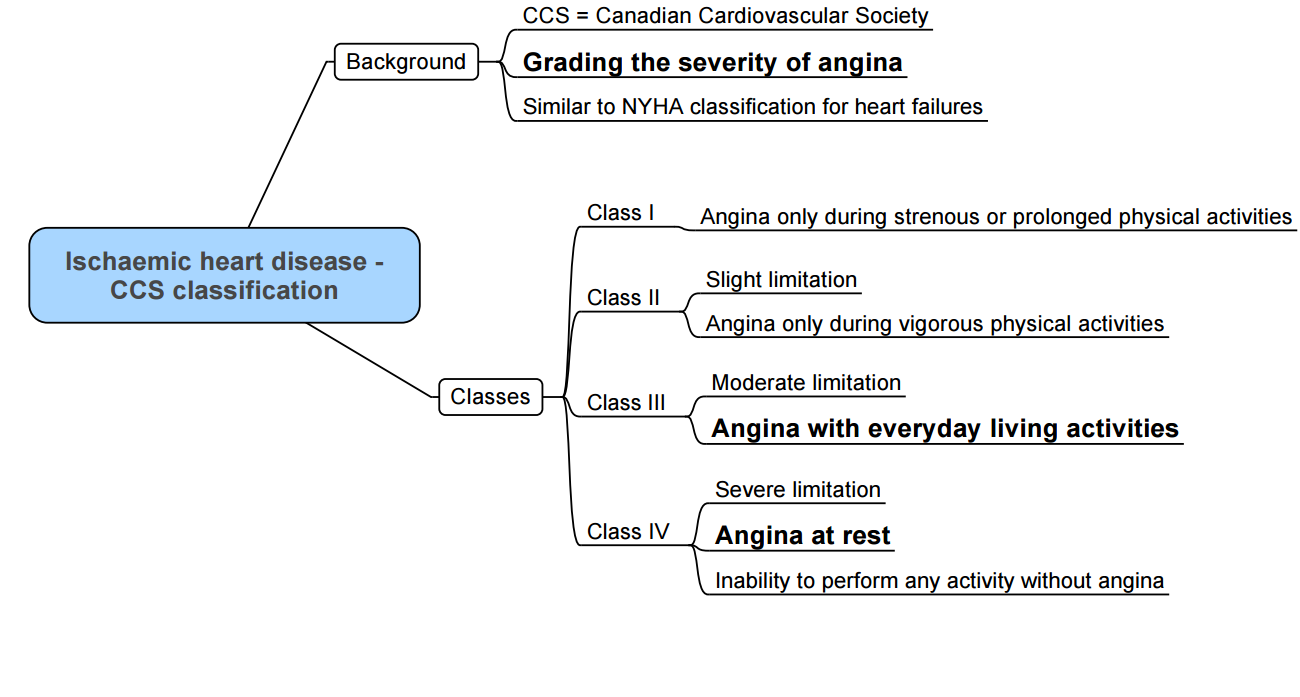
Assessment of Functional Capacity: The Duke Activity Index
- 1 MET (Metabolic Equivalent): Oxygen consumption of 3.5 ml/kg/min.
| Exercise Level | Equivalent Activity |
|---|---|
| 1-4 METs | Standard light home activities, walk around the house, take care of yourself (eating, bathing, using the toilet). |
| 5-9 METs | Climb a flight of stairs, walk up a hill, walk one or two blocks on level ground, run a short distance, moderate activities (golf, dancing, mountain walk), have sexual relations. |
| >10 METs | Strenuous sports (swimming, tennis, bicycle), heavy professional/domestic work such as scrubbing floors, lifting or moving heavy furniture. |
Cardiac Risk Classification of Non-Cardiac Surgical Procedures
- Elevated Risk (> 1%)
- Emergent major operations, particularly in the elderly.
- Aortic and other major vascular surgery.
- Peripheral vascular surgery.
- Anticipated prolonged surgical procedures with large fluid shifts and/or blood loss.
- Carotid endarterectomy.
- Head and neck surgery.
- Intraperitoneal and intrathoracic surgery.
- Orthopedic surgery.
- Prostate surgery.
- Low Risk (< 1%)
- Endoscopic procedures.
- Superficial procedures.
- Cataract surgery.
- Breast surgery.
Pre-Operative Interruption and Resumption of Antiplatelet Therapy
| Agent | Stop Before Surgery | Resume After Surgery | Dose |
|---|---|---|---|
| Oral | |||
| Aspirin | 7 days | 24 h | 80-160 mg daily |
| Clopidogrel | 5 days | 24 h | Load with 300-600 mg, then 75 mg/day |
| Prasugrel | 7 days | 24 h | Bolus 60 mg, then 10 mg/day |
| Ticagrelor | 5 days | 24 h | Bolus 180 mg, then 90 mg bid |
| Intravenous | |||
| Tirofiban | 4-8 h | 4-6 h | 0.1-0.15 µg/kg/min |
| Eptifibatide | 4-6 h | 4-6 h | 2 µg/kg/min |
| Cangrelor | 60-90 min | 4-6 h | 0.75 µg/kg/min |
Anaesthesia For Ischaemic Heart Disease (IHD)
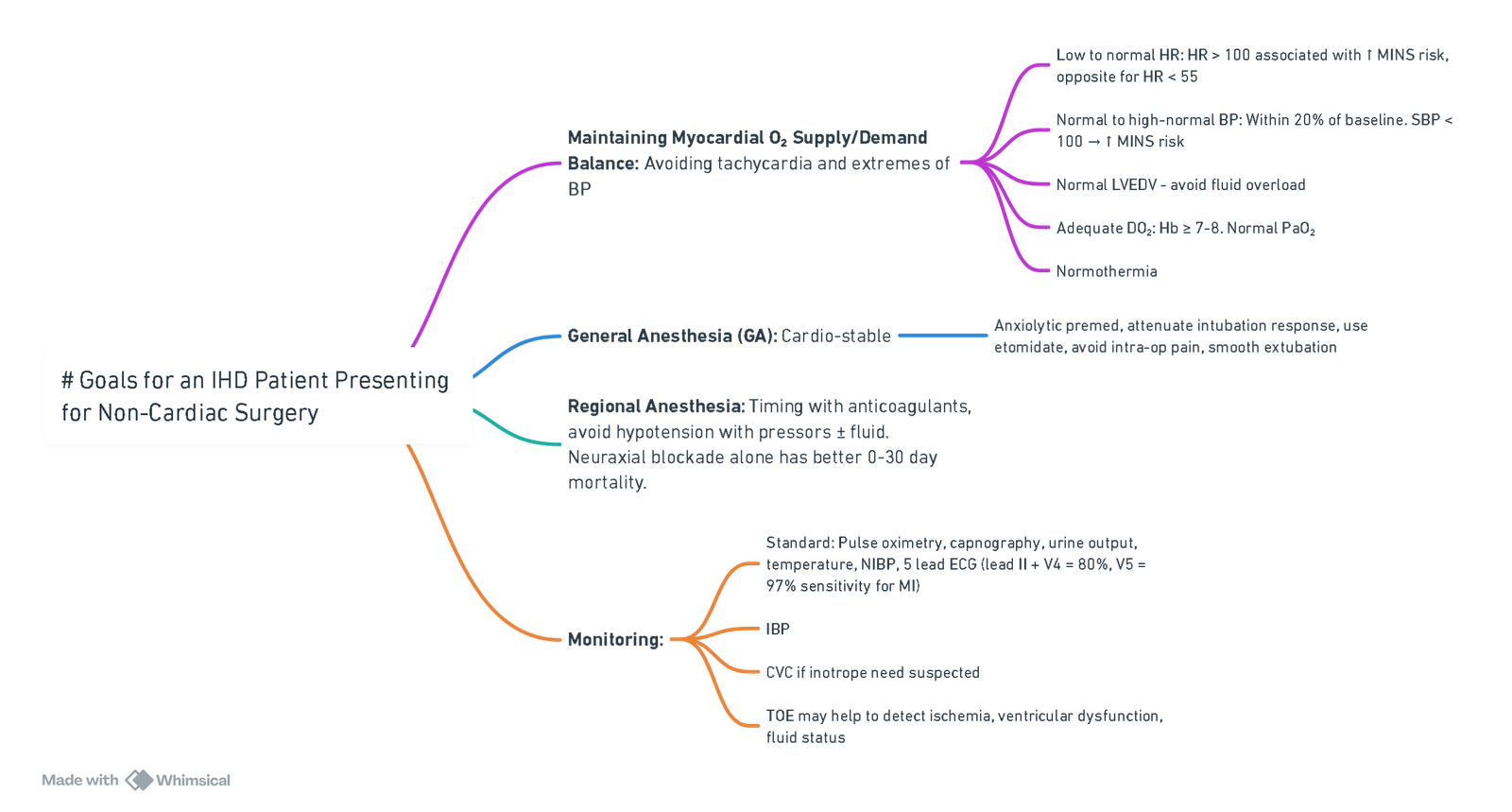
Factors Affecting Myocardial Oxygen Supply-Demand Balance
Factors Decreasing Supply
- Decreased coronary blood flow.
- Tachycardia.
- Hypotension.
- Increased preload.
- Hypoxia.
- Coronary artery spasm.
- Decreased oxygen content and availability (e.g., anemia, hypoxemia).
Factors Increasing Demand
- Tachycardia.
- Increased wall tension.
- Increased afterload (hypertension).
- Increased myocardial contractility.
Goals
- Avoid tachycardia and extreme blood pressure variations.
- Maintain a balance between myocardial oxygen supply and demand.
Intraoperative Management
Premedication
- Use benzodiazepines to reduce anxiety and blunt the stress response.
- Consider etomidate over propofol for induction in hemodynamically compromised patients.
Induction
- Avoid ketamine (as it increases myocardial oxygen consumption).
- Ensure a blunted intubation response using short-acting opioids or beta-blockers.
Maintenance
- Utilize volatile anaesthetics (e.g., isoflurane, sevoflurane) or total intravenous anaesthesia (TIVA) to maintain haemodynamic stability.
Extubation
- Aim for a blunted extubation response to minimize myocardial stress.
- Ensure optimal analgesia and avoid sudden hemodynamic changes.
Monitoring
- Standard Monitoring:
- Pulse oximetry.
- Capnography.
- Non-invasive blood pressure (BP).
- Temperature.
- Urine output.
- Continuous ECG Monitoring:
- Detect myocardial ischaemia and arrhythmias.
- Use computerized ST-segment analysis with multiple lead monitoring (Leads II, V4, and V5).
- Advanced Monitoring:
- Invasive arterial pressure monitoring.
- Central venous catheters.
- Pulmonary artery catheters (if needed for hemodynamic assessment).
- Transesophageal echocardiography (TEE) for ventricular function and regional wall motion abnormalities.
Treatment Of Intraoperative Ischaemia
- Myocardial Oxygen Supply/Demand Balance:
- Deepen the plane of anaesthesia using inhalational or intravenous agents.
- Beta-Blockers:
- Esmolol.
- Metoprolol.
- Labetalol.
- Vasodilators:
- Nitroglycerine for coronary perfusion improvement.
- Hypotension Management:
- Treat with phenylephrine and fluids to maintain coronary perfusion.
- Other Measures:
- Maintain hemoglobin >8 g/dL.
- Treat hypothermia.
- Address arrhythmias promptly.
Postoperative Management
- Cardiac Monitoring:
- Serial 12-lead ECGs to detect ischaemic events.
- Troponin measurements to assess myocardial injury.
- Pain Management:
- Ensure effective analgesia while avoiding significant hemodynamic changes.
- Avoid COX-2 inhibitors to reduce cardiovascular risk.
- Hemodynamic Stability:
- Monitor for signs of recurrent ischaemia, arrhythmias, or myocardial dysfunction.
Peri-Operative Myocardial Infarction (MI)
- May be due to myocardial oxygen supply/demand mismatch or acute plaque disruption.
- Prevention of Peri-Operative MI: Pre-operative coronary revascularization and pharmacological intervention.
Coronary Revascularization Indications
- Acceptable coronary revascularization risk and viable myocardium with left main coronary artery stenosis.
- Three-vessel coronary artery disease with left ventricular dysfunction.
- Left main equivalent (high-grade block in left anterior descending and circumflex arteries).
- Intractable coronary ischemia despite maximal medical therapy.
- Major noncardiac procedures should wait at least 4–6 weeks (possibly 6 months).
- In patients with recent coronary angioplasty and stenting, risk of stent thrombosis and MI increases if dual antiplatelet treatment is stopped; risk of surgical bleeding increases on continuation of drugs.
- Use low-dose aspirin (75 mg/day) based on individual decision considering peri-operative bleeding risk and thrombotic complications.
- ACC/AHA guidelines recommend a delay of at least 6 weeks between bare-metal stent insertion and noncardiac surgery, and 6 months (preferably 1 year) delay for drug-eluting stents for stent reendothelization. In case stent insertion is required before surgery, either bare-metal stent insertion or percutaneous angioplasty is preferable.
Stents
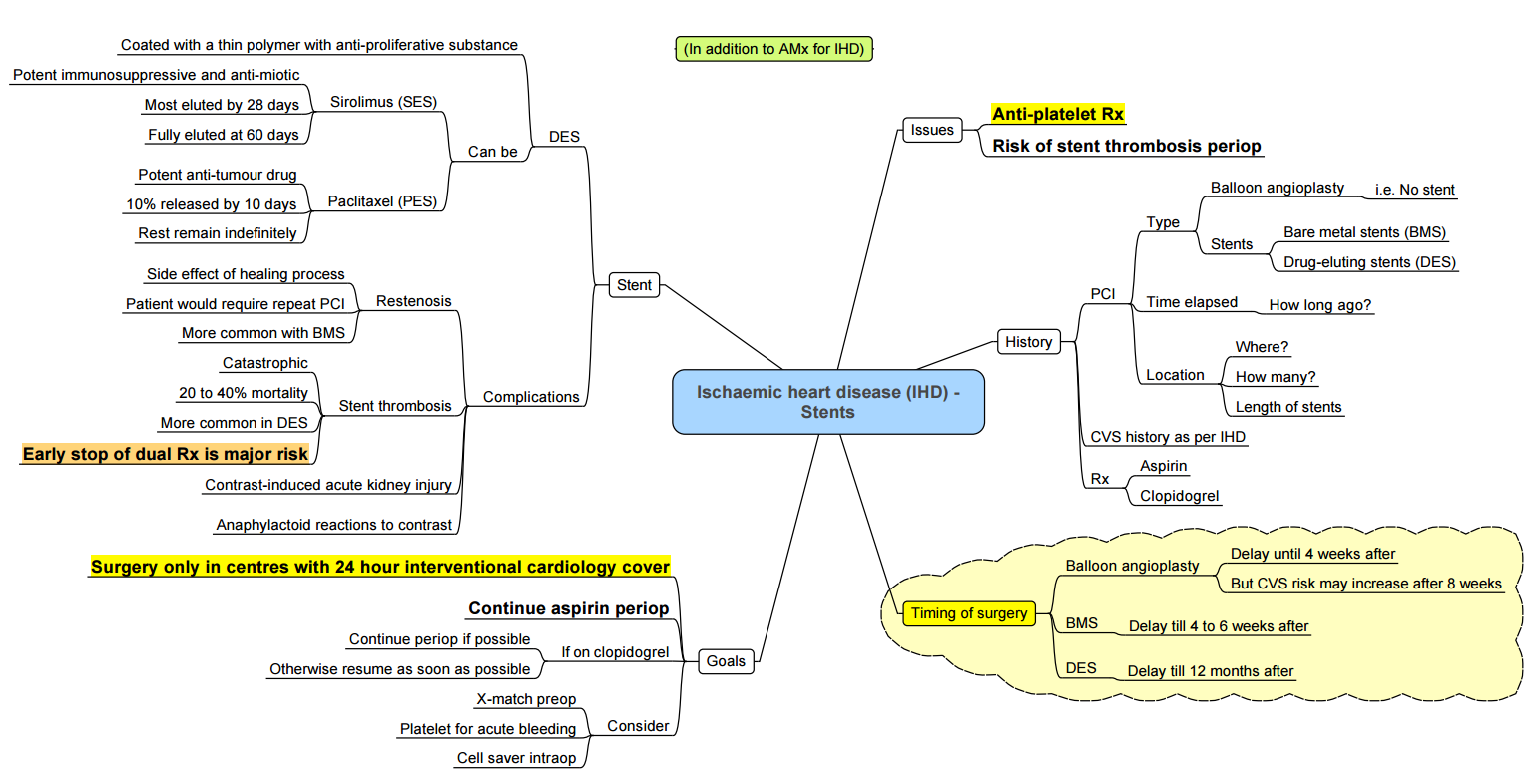
Bridging Therapy
- Drugs for bridging therapy: tirofiban, eptifibatide, and cangrelor.
- Oral antiplatelet drugs are stopped 5–7 days before planned surgical procedures and started on continuous IV infusion of tirofiban or eptifibatide until 4–6 h of procedures. These drugs are restarted postoperatively till DAPT can be reinstituted.
Medical Management
- Peri-Operative Beta-Blockers:
- Metoprolol, atenolol, and bisoprolol are commonly used.
- Target HR: 50–70 bpm.
- ACC/AHA guidelines suggest continuing beta blockers preoperatively and throughout the perioperative period in patients already on them.
- Beta-blockers should be started at least 24 hours before elective surgery and dose titrated to achieve the target HR of 50–60 bpm without significant hypotension.
Types of MI
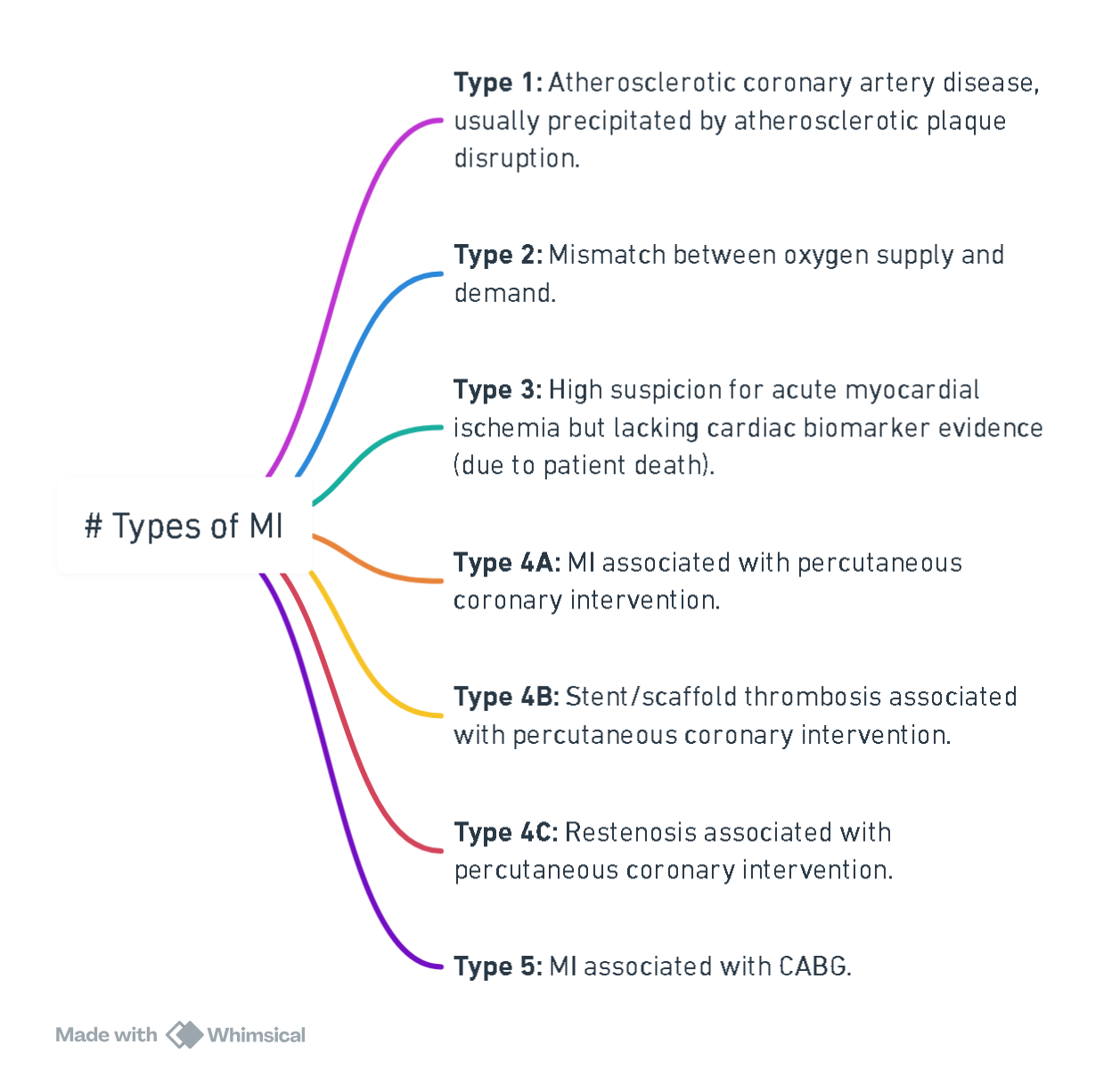
View or edit this diagram in Whimsical.
Links
References:
- The Calgary Guide to Understanding Disease. (2024). Retrieved June 5, 2024, from https://calgaryguide.ucalgary.ca/
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org/
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
- Lees, H. D. and Charlesworth, M. (2021). Anaesthesia for patients with cardiac disease undergoing non-cardiac surgery. Anaesthesia &Amp; Intensive Care Medicine, 22(5), 297-300. https://doi.org/10.1016/j.mpaic.2021.03.008
- Hedge, Jagadish; Balajibabu, PR; Sivaraman, Thirunavukkarasu. The patient with ischaemic heart disease undergoing non cardiac surgery. Indian Journal of Anaesthesia 61(9):p 705-711, September 2017. | DOI: 10.4103/ija.IJA_384_17
Summaries:
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “81c1be4c-89a0-451b-b016-72eb0a19f391”



