- Spinal Anaesthesia
- Introduction
- Advantages of Regional Anaesthesia versus General Anaesthesia
- Benefits of Spinal (neuraxial) Anaesthesia over General Anaesthesia by Surgical Category
- Contra-indications
- Complications
- Functional Anatomy of Spinal Block
- Pharmacology of Intrathecal Local Anaesthetics
- Spread Determinants
- Intrathecal Adjuvants (typical Adult doses)
- Managing Difficult or Failed Spinal Anaesthesia
- Dose guide–single-shot Spinal Anaesthesia in an Average Adult (60–90 Kg, Supine, non-pregnant)
- Different Techniques
- Coagulation
- Epidural
- Functional Anatomy and Physiology
- Anatomic Landmarks to Identify Spinous Processes
- Surface Landmark Correlation to Dermatomal Levels
- Vertebral Column & Curves
- Ligamentum Flavum
- Epidural Space
- Batson Valveless Venous Plexus
- Skin-to-epidural Depth (adult means)
- Dura & CSF Termination
- Sympathetic Outflow & Physiological Consequences
- Drug Spread Determinants (practical rules)
- Catheter Behaviour
- Differential Nerve Susceptibility (why Dilute LA works)
- Benefits & Indications of Epidural Anaesthesia / Analgesia
- Contraindications to Epidural Block
- Epidural Block in Patients Receiving Antithrombotic Therapy
- Epidural Adjuvants, Block Optimisation & Redosing
- Epidural Technique
- Alternative Needle Approaches
- Initiation & Management of an Epidural Block
- Confirming Catheter Position
- Dosing Strategy
- Top-up (redosing)–integrate Pharmacokinetics
- Problem-solving during Placement
- Catheter Management Pearls
- Avoiding Epidural-vein Cannulation
- Relative Order of Peak Plasma Concentration of Local Anesthetic Associated with Regional Anesthesia (Descending Order)
- Functional Anatomy and Physiology
- Spinal Block Failure
- Links
- Past Exam Questions
{}
Spinal Anaesthesia
Introduction
- Advancements in needle design
- Spinal (sub-arachnoid) anaesthesia consists of a single injection of local anaesthetic (± adjuvant) into cerebrospinal fluid, producing rapid, dense sensory, motor and sympathetic block below the intended dermatome.
- Continuous spinal anaesthesia (CSA) fell out of favour in the early 1990s after 5 % lidocaine delivered through micro-catheters was linked to cauda-equina syndrome. Modern CSA, performed with standard epidural catheters and dilute bupivacaine or ropivacaine, is again used selectively (e.g. geriatric hip fracture, major vascular surgery) with good safety when dosing limits are respected
- Modern needle evolution–Greene’s 26 G atraumatic needle (1951) dramatically reduced post-dural-puncture headache (PDPH). Pencil-point needles (Whitacre ➔ Sprotte) further lowered the PDPH rate (< 1 %) and remain standard of care
Advantages of Regional Anaesthesia versus General Anaesthesia
| Benefit | Contemporary evidence & surgical setting | Key findings |
|---|---|---|
| ↓ Post-operative nausea & vomiting (PONV) | Systematic review of “awake” (spinal) vs GA across mixed orthopaedic day-case procedures, 29 RCTs | Spinal technique lowered risk of PONV by ≈35 % (RR 0.65) compared with GA |
| Attenuated surgical stress response | RCT, laparoscopic cholecystectomy (n = 60) | Peak plasma cortisol & glucose were 30–40 % lower after spinal vs GA, indicating blunted neuro-endocrine stress |
| Superior peri- & post-operative analgesia / ↓ opioid use | Multicentre registry, 54 000 total joint arthroplasties | 24 h oral morphine equivalent dose fell by 22 % after spinal compared with GA, with similar pain scores |
| Potential pre-emptive analgesia (↓ chronic pain) | Narrative review of pre-emptive analgesia mechanisms | Early blockade of afferent input with spinal anaesthesia reduces central sensitisation and may limit chronic post-surgical pain development → has been hypothesised to limit CPSP, but meta-analyses show inconsistent benefit outside high-pain surgeries such as thoracotomy or amputation; further RCTs are ongoing |
| ↓ Post-operative pulmonary complications (PPCs) | Propensity-matched cohort of hip- & knee-arthroplasty patients in a fast-track pathway (2023)–“Comparison of pneumonia and major complications after TJA with spinal vs general anaesthesia”. AJRR 2017-20 elective THA registry (> 220 000 cases). |
Spinal/epidural cut 30-day composite PPCs (pneumonia ± re-intubation) from ~1.1 % (GA) to ~0.8 % (spinal)–≈ 25 % relative reduction; matched cohort showed absolute pneumonia fall of 5 % (7.7 %→2.4 %). |
| ↓ Venous thrombo-embolism (DVT ± PE) | Landmark meta-analysis of 141 RCTs across specialties (Rodgers et al., BMJ 2000). AJRR 2017-20 THA registry. |
Neuraxial techniques lowered clinically detected DVT by 44 % and PE by 55 % vs GA in RCTs; contemporary registry shows symptomatic VTE 0.9 % (GA) vs 0.6 % (spinal), adjusted OR 0.78 (≈ 22 % relative reduction). |
| Earlier mobilisation & discharge | Single-centre pathway change to spinal THA (n = 700) | Median hospital length of stay shortened by 0.6 days and proportion of same-day discharge rose from 12 % to 27 % |
| ↓ Intra-operative blood loss and risk of blood transfusions | Direct-anterior THA cohort (n = 412) | Mean blood loss 98 mL lower with spinal vs GA; transfusion rate halved (4 %→2 %) |
| TURP review | Spinal anaesthesia reduced venous pressure and bleeding by ≈80 mL vs GA and allowed earlier detection of TURP syndrome whilst patient awake | |
| Economic saving | Cost-effectiveness analysis across multiple specialities | Spinal anaesthesia 10 % cheaper than GA when drugs, airway equipment and recovery costs included |
| Enhanced communication / positioning | “Awake” lumbar spine surgery series | Real-time feedback enabled safer positioning, fewer pressure-injury complications and improved patient satisfaction |
| Avoidance of airway instrumentation (difficult airway benefit) | Educational review (StatPearls) | Neuraxial blocks eliminate laryngoscopy, advantageous in severe cervical spine disease, ankylosing spondylitis or obesity-OSA syndromes |
| Minimal environmental footprint | Life-cycle analyses & ASA sustainability reports | Spinal techniques avoid volatile agents with high global-warming potential, cutting CO₂-equivalent emissions from ≈22 kg (sevoflurane GA) to <0.1 kg per case |
Benefits of Spinal (neuraxial) Anaesthesia over General Anaesthesia by Surgical Category
| Surgical procedure (population) | Key contemporary evidence (design / n) | ↓ Pulmonary & thrombo-embolic events / blood loss | Analgesia & opioid use | Recovery / LOS / delirium | Economics / Environmental impact | Additional comments |
|---|---|---|---|---|---|---|
| Total hip / knee arthroplasty (primary) | Large US database study 2019-22 | 30-day composite complications ↓ 18 % | Lower OME consumption 24–48 h | Median LOS 0.6 d shorter; ↑ home discharge | Mean CO₂e 63 g vs 22 700 g (GA) in TKA life-cycle analysis | Benefit consistent in fast-track pathways |
| Revision THA / TKA | Multicentre registry 2016-23; 10 554 cases, retro-cohort | — | Pain scores ↓ 1.2 V A S; OME ↓ 27 % | LOS 0.8 d shorter; ↓ ICU admission 22 % | — | Lower 90-day readmission & re-revision rates |
| Hip-fracture surgery (elderly) | REGAIN RCT 2021; n = 1 600 | Equivalent serious complications; obs. data show ↓ pneumonia / PE | — | Equivalent walking recovery; trend to ↓ early delirium in subgroup analyses | — | Choice should consider anticoagulation, cognition |
| Caesarean delivery | Narrative review & cohort syntheses 2023-24 | No airway manipulation → ↓ aspiration / hypoxaemia | Excellent intra- & postoperative analgesia (intrathecal morphine) | Lower PONV, faster bonding & breastfeeding | Lower drug & gas cost; negligible GHG | Gold-standard technique unless GA indicated |
| Transurethral resection of prostate (TURP) | Systematic review + NSQIP analysis 2019-21 | ↓ Venous air / embolism; blood loss ↓ 80 mL; early TURP-syndrome detection | — | Shorter PACU stay; ↓ 30-day mortality 0.4 → 0.1 % | — | Useful when fluid absorption monitoring is critical |
| Lower-limb revascularisation | Systematic review 2022; 18 studies, 5 312 pts | Fewer pulmonary complications, ↓ AKI incidence | — | Shorter ICU & hospital LOS (≈ 1 day) | — | Consider if anticoagulation allows |
- Abbreviations: OME = oral morphine equivalent; LOS = length of stay; CO₂e = carbon-dioxide equivalent; PE = pulmonary embolism; PACU = post-anaesthetic care unit; AKI = acute kidney injury.
Neuraxial Vs General Anaesthesia—contemporary Perspective (2025)
Context
- Pre-2000 studies pre-dated LMWH prophylaxis, lung-protective ventilation, multimodal analgesia and ERAS pathways, so they over-estimated mortality, DVT and pneumonia benefits.
- Modern, event-powered RCTs (e.g. REGAIN 2021, RAGA 2022) and large registries show only modest absolute risk reductions (≈ 0.3–0.5 %) in pulmonary or thrombo-embolic events.
2. Advantages that Still Matter
| Domain | Contemporary rationale |
|---|---|
| PONV & opioid-sparing analgesia | Volatile avoidance plus dense afferent block; benefit remains independent of GA refinements. |
| Environmental & cost savings | No volatile agents → CO₂-equivalent emissions < 1 % of sevoflurane GA; ~10 % lower direct theatre costs. |
3. Areas where the Gap Has Narrowed
- Pulmonary complications: lung-protective ventilation, CPAP and early mobilisation cut baseline risk; absolute advantage of neuraxial now < 1 % in most elective arthroplasty patients.
- VTE: universal chemoprophylaxis and early weight-bearing lower baseline DVT/PE; neuraxial adds only 0.2–0.4 % absolute risk reduction.
- Chronic post-surgical pain: convincing benefit limited to high-pain operations (thoracotomy, amputation); evidence inconsistent for routine joint arthroplasty.
4. Mortality and Long-term Outcomes
- REGAIN and RAGA demonstrate equivalent 60-day mortality and delirium in hip-fracture patients; benefit is procedureand risk-group-specific rather than universal.
- Earlier meta-analyses (e.g. Rodgers 2000) reported ~30 % mortality reduction, but these figures are not reproducible under modern peri-operative care.
5. Practical Implications
- Use neuraxial by default when: high-nociception surgery, difficult airway, high pulmonary/VTE risk, or environmental sustainability is prioritised and coagulation status allows.
- Prefer GA with regional adjuncts when: anticoagulation cannot be interrupted, rapid turnover is critical, or profound sympathectomy is haemodynamically risky.
- Always present current, procedure-specific absolute risks to patients; shared decision-making should weigh marginal clinical gains against logistical constraints and individual comorbidity.
Regain and RAGA
| Trial (year) | Design & population | Primary endpoint (time-frame) | Core findings | How this contrasts with “classic” neuraxial literature |
|---|---|---|---|---|
| REGAIN (NEJM 2021) | Pragmatic, superiority RCT; 1 600 previously ambulant patients ≥ 50 y having hip-fracture repair at 46 US/Canadian hospitals; spinal (no heavy sedation) vs modern GA (volatile ± TIVA) | Composite of death or failure to walk ≥ 3 m independently at 60 days | Primary Endpoint: Spinal 18.5 % vs GA 18.0 % (RR 1.03, 95 % CI 0.84–1.27); 60-d mortality: 3.9 % vs 4.1 % delirium 20.5 % vs 19.7 %; LOS and serious complications similar. |
Earlier meta-analyses (e.g. Rodgers 2000) suggested ≈30 % mortality reduction and lower pneumonia/VTE. REGAIN, under ERAS care and mandatory thromboprophylaxis, found no survival, mobility or delirium advantage. |
| RAGA (JAMA 2022) | Multicentre, open-label RCT; 950 patients ≥ 65 y (10 % dementia) in 9 Chinese teaching hospitals; regional (spinal/epidural without any sedation) vs modern GA | Post-operative delirium within 7 days (CAM-C) | Delirium: regional 5.5 % vs GA 6.0 % (RR 0.92, 95 % CI 0.63–1.35) 30-d mortality 1.5 % vs 1.5 % no differences in complications, pain or LOS. |
Classic observational data linked neuraxial anaesthesia to markedly lower delirium; RAGA shows that when depth-of-anaesthesia and analgesia are well controlled, the delirium gap disappears. |
- Key take-aways
- Both trials were powered for hard outcomes and conducted with contemporary standards—lung-protective ventilation, multimodal analgesia, early mobilisation and routine LMWH.
- They demonstrate that the once-heralded large mortality, pulmonary and thrombo-embolic advantages of spinal/epidural are largely attenuated in modern practice.
- Neuraxial techniques still bring meaningful gains in PONV reduction, early analgesia/opioid sparing and environmental impact, but clinicians should no longer expect dramatic differences in survival, delirium or mobility solely from choice of anaesthetic mode; patientand procedure-specific factors now drive the decision.
Contra-indications
| Absolute | Key evidence |
|---|---|
| Patient refusal, inability to cooperate | Ethical & legal requirement (ASA guidance) |
| Infection at puncture site or systemic bacteraemia | ↑ meningitis / epidural abscess risk 0–0.04 % |
| Uncorrected severe hypovolaemia / shock | Exacerbates 15–75 % hypotension rate |
| Raised intracranial pressure with focal mass | Danger of brain herniation |
| Untreated coagulopathy or therapeutic anticoagulation (platelets < 70 × 10⁹ L⁻¹, INR > 1.4, anti-Xa within dosing window) | Haematoma risk rises from 0.02 % to 0.2 % |
| True allergy to local anaesthetic or preservative | Anaphylaxis case reports |
| High-risk / near-absolute (case-by-case with invasive monitoring): Fixed output states Critical AS/MS, severe pulmonary hypertension or Eisenmenger physiology, LVAD |
| Relative | Typical consideration |
|---|---|
| Sepsis after source control & antibiotics | Proceed with caution once haemodynamics optimised |
| Moderate thrombocytopenia 80–100 × 10⁹ L⁻¹ | Individual risk–benefit with haematology input |
| Evolving neurological disease (MS, spinal stenosis) | No convincing evidence of harm, yet consent carefully |
| Severe anatomical abnormality / previous fusion | May require US or abandon |
Complications
Interpretations of Widely-used Pharmacovigilance Conventions (EMA / WHO / MHRA)
| Term (EMA / WHO) | Numerical band | Everyday wording |
|---|---|---|
| Very common | ≥ 10 % (≥ 1: 10) | Happens to ≥ 1 in 10 patients |
| Common | 1–10 % (≥ 1: 100 < 1: 10) | 1–10 in 100 |
| Uncommon | 0.1–1 % (≥ 1: 1 000 < 1: 100) | 1–10 in 1 000 |
| Rare | 0.01–0.1 % (≥ 1: 10 000 < 1: 1 000) | 1–10 in 10 000 |
| Very rare | < 0.01 % (< 1: 10 000) | < 1 in 10 000 |
Summary of Spinal Complications
- Very common (≥ 10 %)–hypotension, nausea, shivering, pruritus, urinary retention.
- Common (1–10 %)–bradycardia, failed/partial block, PDPH with cutting needle, transient neurological symptoms with lidocaine.
- Uncommon (0.1–1 %)–PDPH with 27 G atraumatic needle, TNS with bupivacaine.
- Rare (0.01–0.1 %)–high/total spinal, hearing impairment, cardiac arrest.
- Very rare (< 0.01 %)–spinal/epidural haematoma, meningitis, abscess, cauda-equina syndrome, permanent needle trauma.
| Category | Complication | Incidence | Principal risk factors | Key prevention / management |
|---|---|---|---|---|
| Very common | Hypotension | 39–75 % (CS) 5–30 % (non-obst) |
High block, fasted, age > 40 y | Co-load 10–15 mL kg⁻¹, prophylactic NE 0.07 µg kg⁻¹ min⁻¹ |
| Nausea ± vomiting | 36 % pooled prevalence (CS) | Hypotension, uterotonics | Maintain MAP, ondansetron 4 mg | |
| Shivering | 52 % (CS) 40–60 % mixed cases |
Low OR temp, intrathecal fentanyl absence | Pre-warm, meperidine 0.25 mg kg⁻¹ | |
| Pruritus (IT morphine) | 43–80 % dose-dependent | IT morphine > 100 µg | Ondansetron / nalbuphine | |
| Urinary retention | 5–25 % ortho / lower-limb surgery | Male sex, opioids, large IV fluids | Bladder scan, early catheterisation | |
| Common | Bradycardia (<50 min⁻¹) | 0.7–15 % | High vagal tone, BJR | Atropine 0.5 mg IV |
| Failed / inadequate block | Complete failure 0.8 %, partial 4–7 % | Technical error, severe scoliosis | Repeat at new space, adjust dose | |
| Uncommon | PDPH | 0.3 % (27 G atraumatic) 1.7 % obstetric Whitacre 27 G |
Young female, large needle | Epidural blood patch 15–20 mL |
| Transient neurological symptoms (TNS) | Lidocaine 8–22 %; bupivacaine ≤0.3 % | Hyperbaric/iso-baric lidocaine | Prefer chloroprocaine / bupivacaine | |
| Rare | High / total spinal | 1:4336 CS; 1:29 770 UK data | Excess dose, short stature | Airway preparedness, vasopressor support |
| Hearing impairment | <1 %; mostly transient case reports | CSF leak | Treat PDPH, blood patch | |
| Cardiac arrest | ~6 / 100 000 anaesthetics (0.006 %) | Young healthy, high block, beta-blocker | Vigilant monitoring, early epinephrine | |
| Very rare | Spinal / epidural haematoma | 0.02–0.03 % (1:33 000–1:50 000); ↑10-fold with anticoagulant breach | Anticoagulants, thrombocytopenia | Strict ASRA timing; urgent MRI + decompression within 8 h |
| Bacterial meningitis | 2.5 / 100 000 obstetric blocks | Breach of asepsis | 0.5 % chlorhexidine–alcohol dry time ≥2 min | |
| Epidural / spinal abscess | 1:63 000–1:145 000 | Diabetes, long catheter dwell | Early MRI, surgical drainage | |
| Cauda equina syndrome | 1: 20 000–>100 000; linked to 5 % lidocaine micro-catheters | High concentration local anaesthetic | Use single-dose > avoid micro-catheter lidocaine | |
| Permanent needle trauma / spinal cord ischaemia | ≤1: 90 000 in modern series | High or repeated attempts, severe stenosis | Ultrasound guidance, gentle technique |
- Incidence bands reflect the best available population or meta-analysis data 2018-2025.
- Small calibre need can reduce PDPH to < 1 % in most non-obstetric patients; in obstetrics the incidence remains ~1–2 % even with 25–27 G pencil-point needles, so vigilance and counselling are still required.
- Complications of Spinal Anaesthesia
Specific Complications
Cardiovascular Effects
- Hypotension remains the commonest adverse event; NE infusion 0.07 µg kg⁻¹ min⁻¹ offers the best balance of preventing hypotension without excess hypertension in CS meta-analysis.
-
- Maintain MAP ≥ 80 % baseline; use NE (preferred) or PE infusions titrated with continuous non-invasive BP monitoring.
-
- Bezold-Jarisch bradycardia: occurs in ≈15 % of mixed surgical cohorts; ondansetron (4–8 mg IV) pre-spinal reduces incidence by antagonising 5-HT₃-mediated vagal activation.
- Ondansetron blunts 5-HT₃ receptors in cardiac vagal fibers, significantly decreasing episodes of bradycardia and hypotension during spinal anesthesia.
Cauda Equina
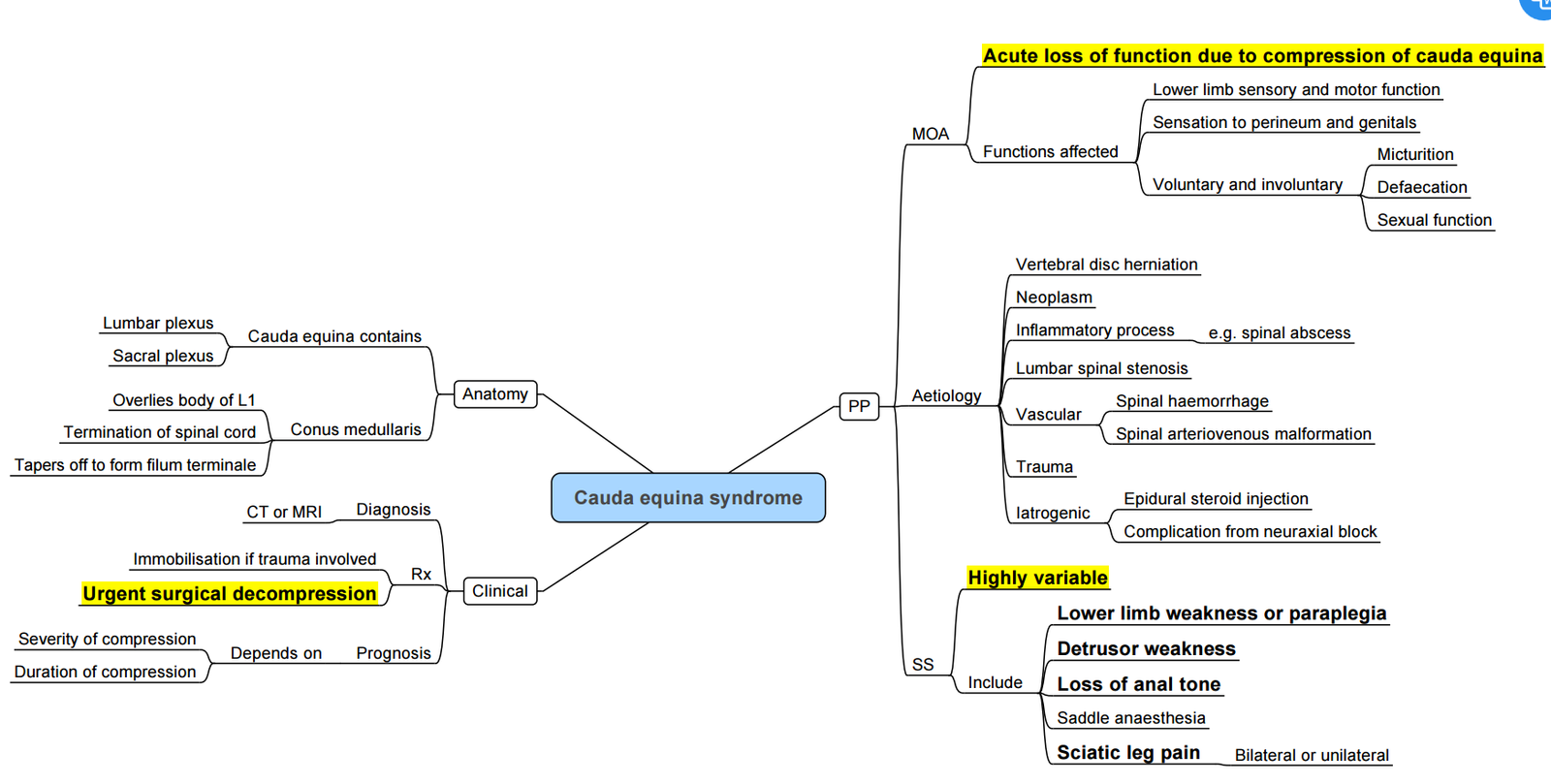
Functional Anatomy of Spinal Block
| Component | Key facts & figures | Clinical relevance |
|---|---|---|
| Vertebral column | 33 vertebrae (7 C, 12 T, 5 L, 5 S (fused), 4 Co). Normal curvatures: cervical & lumbar lordosis, thoracic kyphosis. | Lumbar lordosis ↑ skin–dura distance; flatten with hip flexion to widen interlaminar spaces. |
| Ligaments | • Supraspinous (tips C7–S1) • Interspinous (between spines) • Ligamentum flavum (paired, elastic, 3–5 mm thick in L region) • Posterior & anterior longitudinal |
Distinct “pop” when needle pierces ligamentum flavum; thickness explains loss-of-resistance depth correlation. |
| Spinal cord termination | Conus medullaris ends most commonly at mid-L1 (range T12–L3); <1 % lie below L2/3. | Safe lumbar puncture window = L3/4 or lower. Neonates: conus at L3 and sac termination lower–choose L4/5. |
| Dural sac termination | S2 in 82 % of adults (range S1–S4). | Avoid intentional dural puncture below S2 during caudal blocks. |
| Subarachnoid (intrathecal) space | Contains ≈ 60–80 mL CSF in lumbar cistern; volume falls by ≈ 30 % in late pregnancy / obesity. | ↓CSF volume → greater cephalad spread for same LA dose; reduce dose 20–30 % in term parturient. |
| Blood supply | Single anterior spinal artery (ASA, supplies ant 2/3 cord) + paired posterior spinal arteries; reinforced by segmental radicular vessels, largest = artery of Adamkiewicz (T8–L2). | Severe, prolonged hypotension or vasoconstrictors may cause ASA ischaemia → paraplegia; avoid high vasopressor doses. |
| Distances (skin → epidural → dura) | Mean skin-epidural depth at L3/4 ≈ 4.5 cm (range 2.5–8 cm) in adults; ultrasound predicts depth within ±5 mm. | Anticipate needle length; difficult in obesity–handheld ultrasound improves first-pass success. |
| Needle trajectory | Midline: skin → subcutaneous fat → supraspinous → interspinous → ligamentum flavum → epidural → dura → arachnoid → CSF. Paramedian: skin 1 cm lateral → paraspinal muscle → ligamentum flavum → as above |
Paramedian bypasses calcified interspinous ligaments in elderly. |
| Subdural space | “Potential” space between dura & arachnoid; capacity < 2 mL. More compliant dorsally than ventrally → preferential dorsal spread sparing motor fibres. | Accidental subdural injection: high sensory block, minimal motor / sympathectomy; resolves < 2 h. |
| Surface landmarks | Inter-cristal (Tuffier’s) line = L4 spinous process/inter-space. Palpate midline spines; ultrasound if impalpable. | Choose L3/4 or L4/5 for spinal puncture. |
| Dermatome targets | • T4 nipple line–upper abdominal surgery • T6–intestinal/ gynae/ urology • T10–TURP, vaginal delivery, hip • L1–thigh • L2–knee/foot • S2–S5–perineum (“saddle”) |
Match desired sensory level to drug dose ± baricity and patient position. |
Pharmacology of Intrathecal Local Anaesthetics
Key Factors
- Potency: Related to lipid solubility—higher lipid solubility increases potency, allowing for effective analgesia at lower concentrations.
- Duration of Action: Influenced by protein binding—higher protein binding results in a longer duration of action.
- Onset of Action: Related to the amount of local anesthetic available in its base form. Lower pKa values correspond to a faster onset of action.
Pharmacokinetics in the Subarachnoid Space
- The uptake of local anaesthetics into neuronal tissue from the subarachnoid space depends on:
- Concentration of the anaesthetic in CSF
- Surface area of nerve tissue exposed to CSF
- Lipid content of nerve tissue
- Blood flow to nerve tissue
Mechanisms of Uptake
- Local anesthetics are absorbed by nerve roots and the spinal cord. The spinal cord absorbs anesthetics via diffusion from the CSF and through the spaces of Virchow-Robin, which connect with perineuronal clefts and allow deeper penetration into the spinal cord. The distribution of local anesthetics is influenced by baricity, patient positioning, and dose.
| Variable | Bupivacaine | Ropivacaine | Lidocaine | Chloroprocaine |
|---|---|---|---|---|
| pKa | 8.1 | 8.1 | 7.8 (fast onset) | 8.9 |
| Lipid solubility (relative) | High | Moderate | Low | Very low |
| Protein binding | 95 % | 94 % | 64 % | 0 % |
| Differential block | Dense sensory & motor | Sensory > motor (good for day-case) | Non-selective; ↑ TNS | Minimal motor; rapid regression |
| Neuro-toxicity | Cardiotoxic if intravascular | Lower cardio-toxicity | ↑ TNS >50 mg | Safest for short cases |
- Order of nerve fibre block: B (fast pre-ganglionic) → C & A-δ (pain/temp) → A-γ (proprioception) → A-β (touch) → A-α (motor).
Spread Determinants
| Determinant | Effect size / comment |
|---|---|
| Baricity (ρ) | Hyperbaric (ρ > CSF) sinks with gravity; hypobaric rises; isobaric remains at injection level. A 0.0006 density change shifts median sensory level ≈ 3 dermatomes. |
| Dose | Each additional 0.5 mL of 0.5 % hyperbaric bupivacaine raises block ≈ 3 dermatomes. |
| CSF volume | 10 mL decrease (e.g. pregnancy / obesity) → 2–4 dermatomes higher block; adjust dose ↓ 20–30 %. |
| Patient position | Trendelenburg after hyperbaric dose promotes cephalad spread; reverse Trendelenburg or sitting limits it. |
| Injection speed | Inject the dose steadily (≈15–20 s for 2.5 mL) to minimise turbulence; evidence for a strict rate threshold is limited. |
| Bevel orientation | In lateral decubitus, bevel “down” with hyperbaric LA favours unilateral block. |
| Injection force / pressure | High-pressure (> 15–20 psi) injection associated with irregular, higher cephalad spread and ↑ risk of high spinal; use gentle steady pressure. |
| Vasoconstrictor addition (e.g. epinephrine 1:200 000) | Negligible impact on block height; prolongs duration 15–30 % (mainly with lidocaine/chloroprocaine). Very high concentrations theoretically ↓ spinal cord blood flow. |
Elimination
- Local anaesthetic is removed by:
- Vascular uptake–epidural veins dominate (tetracaine > bupivacaine > lidocaine).
- CSF bulk flow–0.3 mL min⁻¹ cranio-spinal circulation.
- Systemic redistribution–hepatic metabolism (amide agents) or plasma cholinesterase (esters e.g. chloroprocaine).
Intrathecal Adjuvants (typical Adult doses)
| Class | Dose | Effect | Caveats |
|---|---|---|---|
| Morphine (preservative-free) | 100–200 µg | 18–24 h analgesia | Itch, N/V, delayed resp depression |
| Fentanyl | 10–25 µg | Fast onset, improves block density | Synergistic PONV |
| Dexmedetomidine | 5 µg | ↑ sensory & motor block by 90–120 min, ↓ shivering | Mild hypotension / bradycardia |
| Clonidine | 15–30 µg | Similar to dexmedetomidine | Sedation, bradycardia |
| Epinephrine 1:200 000 | 0.1–0.2 mg | Prolongs lidocaine / chloroprocaine only | Theoretical spinal cord ischaemia at high dose |
SASRA Guidelines on Adjuvant Agents in Regional Anaesthesia
-
Adrenaline:
- Prolongs the duration of the blockade when combined with lignocaine only.
-
Clonidine:
- Extends the duration of analgesia and anaesthesia by approximately 2 hours when added to local anaesthetics for axillary and peribulbar blocks.
- Caution: Evidence is inconclusive when clonidine is added to supraclavicular brachial plexus blocks or continuous catheter techniques.
- Side Effects: Dose-independent systemic side effects such as hypotension, bradycardia, and sedation.
- Clonidine in lignocaine intravenous regional anaesthesia (IVRA) delays tourniquet pain.
-
Magnesium Sulphate:
- Improves intra and postoperative analgesia and tourniquet tolerance in lignocaine IVRA.
- The long-term effects of perineural magnesium are unclear.
-
Ketamine:
- Reduces pain when applied topically in oral mucositis.
- Not recommended for addition to local anaesthetic agents.
-
Alkalinisation:
- Addition of sodium bicarbonate is no longer recommended.
-
Dexamethasone:
- Increases the duration of the blockade in peripheral nerve blocks depending on the site of administration, with the most benefit seen with long-acting blocks (50-240% increase).
- The effect is similar if administered intravenously in doses of at least 10 mg.
-
General Advice:
- Some adjuvant agents may be associated with potentially undesirable effects.
- Increased risk of drug errors when combining these adjuvant agents with local anaesthetics.
Common Additives
- Preservative-free morphine 50mcg to 250mcg *(>150mcg = high dose) = prolong postoperative analgesia for 18 to 24 hours. Risk benefit: puritis vs analgesia.
- Recent reports indicate that 5mcg dexmedetomidine added to hyperbaric bupivacaine potentiates and prolongs spinal anaesthesia without any untoward effects on neonate and hence can be used when it is appropriate.
Dexmedetomidine as an Adjuvant in Regional Anesthesia
- Emerging/Investigational evidence: Small RCTs suggest 3–5 µg dexmedetomidine prolongs spinal block in C-section without acute neonatal harm. Not yet guideline-endorsed; reserve for trials or informed-consent use
| Route / Application | Dose / Concentration | Evidence Summary | Benefits | Comparisons / Notes |
|---|---|---|---|---|
| Intrathecal (CS, ortho) | 2–4 µg+ 10–13.5 mg 0.75% bupivacaine ± morphine | Liu et al. 2020; Mo et al. 2023: prolonged block (~2h), reduced pain, ED50 ≈ 3.1–5.9 µg | Fast onset, dense and prolonged block, ↓ shivering, ↓ opioid need | Superior to fentanyl for block duration and shivering reduction |
| Epidural Conversion (CS) | 4 µg + 10–20 mL 2% lidocaine ± bicarbonate | Riham et al. 2016: improved onset, analgesia vs epinephrine | Rapid CS conversion, better intra-op analgesia | Comparable or better than epinephrine |
| Epidural Top-Up (2nd Stage) | 2–4 µg + 4 mL 0.1% ropivacaine ± fentanyl OR 2–3 mL 0.2% ropivacaine | Qian et al. 2021; Yang et al. 2020: faster onset, less nausea/shivering vs fentanyl | Improved block efficacy, less shivering and nausea | Faster onset and fewer side effects than fentanyl |
| Continuous Epidural Infusion | 0.3–0.5 µg/mL in 0.1% ropivacaine | Wei et al. 2022; Pang et al. 2022: fewer PCEA boluses, lower LA use, optimal dose = 0.3 µg/mL | Longer duration, fewer PCEA uses, less pruritus | Better tolerability than sufentanil |
| Peripheral NB (Outpatient) | 0.5–1 µg/kg + local anesthetic | Dai et al. 2018: prolonged block (~3–4h), better analgesia | Faster onset, prolonged analgesia, opioid-sparing | ↑ bradycardia/hypotension vs ropivacaine alone |
| Peripheral NB (Inpatient) | 1–2 µg/kg + local anesthetic | Packiasabapathy et al. 2017; Jung et al. 2018: 2 µg/kg superior to 1 µg/kg for duration (~20h) | Longest block duration (~20h), ↓ postop opioid use | 2 µg/kg better than 1 µg/kg; ↑ hypotension risk |
| Nebulized (PDPH) | 1 µg/kg diluted to 4 mL saline, q12h | Kumar et al. 2019; Mowafy et al. 2021: ↓ PDPH severity, increased CSF pressure, minimal side effects | Non-invasive PDPH relief, minimal sedation | Alternative to conservative PDPH management |
Managing Difficult or Failed Spinal Anaesthesia
- Failure of Spinal Block:
- Failures can occur due to issues with lumbar puncture, solution injection, solution spread in the CSF, drug action on the nerves, or patient management. Correct positioning, anatomical landmark identification, and experience are crucial for successful neuraxial blocks.
- Failed Injection or Spread:
- Causes include loss of injectate during connection or into adjacent tissues, spinal deformities, septae, stenosis, or extradural cysts. Changes in patient position or repeating the block with adjustments are possible remedies, but care must be taken to avoid excessive spread or accumulation of anesthetic.
- Inadvertent Subdural Nerve Block:
- This occurs when the needle unintentionally enters the subdural space. It may present as a high sensory block with motor and sympathetic sparing, or more severe symptoms like respiratory insufficiency or unconsciousness. The block usually resolves within 2 hours.
- Unilateral Spinal Nerve Block:
- Preferred in elderly trauma patients or outpatient surgeries, unilateral spinal anesthesia offers better hemodynamic stability. For elderly patients, a hypobaric solution with the operative side up is used, while in outpatient settings, hyperbaric bupivacaine with the operative side down is recommended. A slow injection rate is critical to achieving a unilateral block.
Dose guide–single-shot Spinal Anaesthesia in an Average Adult (60–90 Kg, Supine, non-pregnant)
| Target sensory level (dermatome) | Typical operations | Hyperbaric bupivacaine 0.5 % (mg / mL) | Isobaric ropivacaine 0.75 % (mg / mL)† | Chloroprocaine 3 % (mg / mL)‡ |
|---|---|---|---|---|
| S2–S5 (saddle) | Perianal fistula, haemorrhoidectomy, obstetric perineal repair | 4–6 mg (0.8–1.2 mL) | 5–7 mg (0.7–1.0 mL) | 30–40 mg (1.0–1.3 mL) |
| L2 | Foot & ankle surgery, knee arthroscopy, short below-knee fracture fixation | 8–10 mg (1.6–2.0 mL) | 10–12 mg (1.3–1.6 mL) | 40–50 mg (1.3–1.7 mL) |
| L1 | Hip nailing, thigh soft-tissue procedures, inguinal hernia repair | 10–12 mg (2.0–2.4 mL) | 12–15 mg (1.6–2.0 mL) | 50–60 mg (1.7–2.0 mL) |
| T10 | TURP, vaginal delivery (labour analgesia top-up), elective hip arthroplasty | 12–14 mg (2.4–2.8 mL) | 15–18 mg (2.0–2.4 mL) | 60–70 mg (2.0–2.3 mL) |
| T6 | Lower abdominal, laparoscopic gynaecology, appendicectomy, renal surgery | 14–16 mg (2.8–3.2 mL) | 18–22 mg (2.4–2.9 mL) | Not recommended (>60 min likely) |
| T4 | Caesarean section, open upper-abdominal surgery, thoraco-lumbar spine | 15–18 mg (3.0–3.6 mL)§ | 20–24 mg (2.7–3.2 mL) | — |
- Reduce dose by ~25 % in term pregnancy, obesity or age > 70 y (↓ CSF volume).
† Ropivacaine produces a slightly less dense motor block–useful for fast-track joint surgery.
‡ Chloroprocaine gives ≤ 60 min anaesthesia–ideal for ambulatory cases; avoid in glucose-6-phosphate dehydrogenase deficiency. - For Caesarean section add intrathecal morphine 100 µg or fentanyl 15 µg for postoperative analgesia.
- These dose ranges align with ED95 data and large clinical series (Miller’s Anaesthesia, 2020; Kokri et al. BJA 2023; De Oliveira et al. Anesth Analg 2022).
Short-acting Spinal Local-Anaesthetic Options
| Drug | Concentration / dose* | Surgical duration | Advantages | Caveats |
|---|---|---|---|---|
| 2 % Hyperbaric Prilocaine | 40–60 mg (2.0–3.0 mL) | 60–90 min | • Rapid onset (≈3 min) • Very low TNS incidence (<0.5 %) • Fast motor recovery–ideal ambulatory joints, cystoscopy |
• Avoid in methaemoglobinaemia risk (G6PD deficiency, infants). |
| 3 % Chloroprocaine | 40–60 mg (1.3–2.0 mL) | 40–60 min | • Fastest offset (<1 h) • Minimal neuro-toxicity • Safe in breast-feeding (rapid hydrolysis) |
• Slightly higher failure if surgery prolonged; ensure theatre efficiency. |
| 1.5 % Hyperbaric Lidocaine (legacy) | 60–75 mg | 45–60 min | • Widely available | • TNS 8–22 %–generally avoided; reserve for proven prilocaine / chloroprocaine shortage. |
*Adult 60–90 kg, seated → supine. Reduce dose ~20 % in term pregnancy / age > 70 y.
Different Techniques
Combined Spinal–Epidural (CSE)
| Aspect | Key points |
|---|---|
| Definition | Single‐interspace technique using a special (needle-‐through–needle) or separate-needle approach: a spinal dose is given, then an epidural catheter is threaded for extension or postoperative analgesia. |
| Pros | • Rapid, reliable onset of dense block (spinal) plus titratable duration (epidural). • Lower total intrathecal dose needed → less hypotension. • Epidural top-ups rescue partial/short blocks, reduce GA conversion. |
| Cons / Cautions | • Two punctures of the dura increase PDPH risk vs spinal alone (still < cutting needles). • Epidural catheter may perforate the fresh dural hole → unrecognised intrathecal catheter. • Slightly longer set-up time; cost of specialised kits. |
| Obstetric ubiquity | CSE is now the preferred technique for elective & urgent caesarean section in many high-volume obstetric units: fast surgical anaesthesia (<5 min) and ongoing epidural analgesia in theatre/PACU. Labour analgesia “walking CSE” (low-dose bupivacaine + fentanyl) combines rapid pain relief with mobilising epidural top-ups. |
Dural-Puncture Epidural (DPE)
| Feature | Summary |
|---|---|
| Technique | Identical to CSE until the spinal needle reaches CSF; no intrathecal drug is injected, the needle is withdrawn and an epidural catheter threaded. 10–25 G spinal needles most used. |
| Rationale | Microscopic dural hole (~0.4 mm) enhances spread of epidural solution into CSF and sacral roots → faster, denser analgesia than plain epidural but PDPH risk similar to conventional epidural. |
| Evidence (2018-24) | • Multiple labour RCTs (n≈1 500 total) show quicker onset (≈5 min) and better S2–S4 coverage, fewer top-ups vs epidural. • PDPH incidence remains ≈0.2 %, no increase vs standard epidural; no neonatal disadvantage reported. |
| Current status | Increasingly used in high-throughput obstetric units for women with challenging pain control (OP position, obesity) where rapid sacral spread is valuable. |
Pre-puncture Ultrasound for Spinal / Epidural
| Benefit | Typical finding |
|---|---|
| Depth prediction | US (2–5 MHz curved probe) measures skin-to-ligamentum flavum/dura depth to ±5 mm accuracy in lumbar region. |
| First-pass success | Meta-analysis 2022 (>3 000 patients)–first-pass spinal success ↑ 30 % in obesity & difficult landmarks; attempts and passes reduced, PDPH not increased. |
| Landmarks | Identify midline (spinous processes), optimum interspace (L3/4 or L4/5), probe in paramedian sagittal oblique view. Mark midline and needle insertion point before prepping. |
| Indications | BMI > 35 kg m⁻², scoliosis/kyphosis, previous spinal surgery, elderly calcified spines, failed landmark attempt. |
Coagulation
Recommended Time Intervals Before or After Neuraxial Procedure and Epidural Catheter Removal
| Drug | Time Before Neuraxial Procedure or Catheter Removal | Time After Neuraxial Procedure or Catheter Removal | Comments |
|---|---|---|---|
| Aspirin | None | None | |
| NSAIDs | None | None | |
| Clopidogrel | 7 days* | After catheter removal | Per European & Scandinavian guidelines |
| Prasugrel | 7-10 days | 6 hours | Per European guidelines |
| Ticagrelor | 5 days | 6 hours | Per European guidelines |
| Warfarin | 5 days (normal INR) | After catheter removal | |
| Heparin (IV) | 4-6 hours | 1-2 hours | |
| Heparin | |||
| – (SC, BID) | None | None | |
| – (SC, TID) | Not applicable | Before neuraxial procedure | |
| LMWH | |||
| – Prophylactic | 12 hours | 4 hours | FDA recommendation |
| – Therapeutic | 24 hours | 4 hours | |
| Fondaparinux | 36-42 hours | 6-12 hours | Per European guidelines. ASRA recommends against neuraxial procedures in patients on this drug.* If a procedure is necessary after 5 days, a test of platelet function is recommended. |
Recommended Time Intervals Before or After Neuraxial Procedure and Epidural Catheter for New Anticoagulants
| Drug | Half-life | European Guidelines | Scandinavian Guidelines | Five Half-lives |
|---|---|---|---|---|
| Dabigatran | 12-17h; 28h (renal disease) | Contraindicated per manufacturer | Data not available | 85h (4 days); 6 days (renal patients) |
| Rivaroxaban | 9-13 hours | 22-26 hours | 18 hours | 65 hours (3 days) |
| Apixaban | 15.2 +/- 8.5 hours | 26-30 hours | Data not available | 75 hours (3-4 days) |
Recommended Time Intervals for Resumption of Drug After Neuraxial Procedure or Catheter Removal
| Drug | European Guidelines | Scandinavian Guidelines | Liew & Douketis (102); Connolly & Spyropoulos (98) |
|---|---|---|---|
| Dabigatran | 6 hours | 6 hours | 24 hours |
| Rivaroxaban | 4-6 hours | 6 hours | 24 hours |
| Apixaban | 4-6 hours | 6 hours | 24 hours |
Anticoagulation & Peripheral Nerve Blocks
- Peripheral nerve blocks can be performed in patients taking anticoagulants, although no prospective studies have been conducted in this context. The ASRA guidelines for neuraxial procedures are generally recommended for peripheral nerve blocks as well. However, the European Society of Anaesthesiology suggests that the guidelines for neuraxial blocks may not routinely apply to peripheral nerve blocks. In particular, the Austrian Society for Anaesthesiology, Resuscitation and Intensive Care notes that superficial nerve blocks can be safely performed with residual anticoagulation.
- Risks: Cases of psoas and retroperitoneal hematomas have been reported following lumbar plexus and psoas compartment nerve blocks in patients on anticoagulants, despite adherence to ASRA guidelines. Symptoms include pain, tenderness, declining hemoglobin/hematocrit, hypotension, and sensory-motor deficits. Diagnosis is confirmed via CT, with ultrasound as a secondary tool.
- Treatment: Treatment options include surgical consultation, anticoagulation reversal, blood transfusion, and possibly surgical drainage.
Summary
- The guidelines for neuraxial injections should also be applied to lumbar plexus nerve blocks and visceral sympathetic nerve blocks.
- For superficial nerve blocks, ultrasound-guided regional blocks may be safely performed in the presence of residual anticoagulation.
Epidural
- Epidural anaesthesia/analgesia (EDA) entails injection or continuous infusion of local anaesthetic (± adjuvants) into the epidural space at any vertebral level to achieve segmental sensory ± motor block. When compared with systemic opioids or general anaesthesia (GA) alone, well-conducted thoracic or lumbar EDA demonstrably:
- lowers major pulmonary-complication rates after thoracotomy, oesophagectomy and colorectal surgery
- reduces postoperative pneumonia in high-risk COPD patients
- shortens postoperative ileus and mechanical-ventilation duration, and decreases 30-day mortality in multi-rib-fracture cohorts
- The widespread use of EDA in labour, major abdominal, thoracic and lower-limb surgery reflects these benefits.
Functional Anatomy and Physiology
| Structure | Details & relevance |
|---|---|
| Epidural space | Potential space between ligamentum flavum and dura; extends foramen magnum → sacral hiatus. Contents: fat, Batson valveless veins, spinal nerve roots. Negative pressure ≈–1 to–5 cmH₂O (lumbar). |
| Depth (skin → epidural) | Mean 4.5 cm lumbar, 3.2 cm mid-thoracic; ultrasound predicts to ±5 mm. Obesity can exceed 8 cm. |
| Segmental spread | Cephalad/caudad via longitudinal ligaments & fat; volume is principal determinant (≈ 1–2 mL per dermatome in adults). |
| Vascular plexus | Engorges in pregnancy & portal hypertension → ↑ risk of intravascular catheter placement & LAST. |
| Sympathetic chain | Thoracolumbar (T1–L2); thoracic EDA can modulate cardiac sympathetic tone (T1–T5) producing relative bradycardia & afterload reduction. |
Anatomic Landmarks to Identify Spinous Processes
| Anatomic Landmark | Vertebral Level |
|---|---|
| Vertebra prominens | C7 |
| Root of the spine of the scapula | T3 |
| Inferior angle of the scapula | T7 |
| Rib margin | L1 |
| Superior aspect of the iliac crest | L3, L4 |
| Posterior superior iliac spine | S2 |
Surface Landmark Correlation to Dermatomal Levels
| Level of Block | Anatomic Landmark |
|---|---|
| C6 | Thumb |
| C8 | Fifth finger |
| T1 | Inner aspect of the arm |
| T4 | Nipple |
| T6 | Xiphoid process |
| T10 | Umbilicus |
| T12 | Inguinal ligament |
| S1 | Lateral aspect of the foot |
| S2-S4 | Perineum |
Vertebral Column & Curves
- 33 vertebrae: 7 C, 12 T, 5 L, 5 fused S, 3-5 fused Co.
- Lordosis peaks at C5 and L3; kyphosis peaks at T5–T7 and S2.
- Supine flexion of hips/knees flattens lumbar lordosis, widening inter-laminar spaces for easier mid-line needle passage.
Ligamentum Flavum
- 80 % elastin; thickness ↑ caudally (≈1.5 mm cervical → 4–5 mm lumbar).
- Mid-line fusion gap present in 10–15 % of adults (L3–S1)–may abolish the usual “loss-of-resistance pop”.
- Clinical tip–advance slowly with saline loss-of-resistance to avoid false entry.
Epidural Space
- Extends foramen magnum → sacrococcygeal membrane; average antero-posterior width 5–6 mm lumbar, 3–4 mm thoracic.
- Three compartments: posterior (target for needle/catheter), lateral (nerve-root sleeves), anterior (venous plexus).
- Epidural pressure profile–Sub-atmospheric (−2 to −6 cm H₂O) at mid-/upper-thoracic levels, providing the basis for a reliable hanging-drop sign there. Lumbar pressures are usually zero or slightly positive; therefore the hanging-drop test is unreliable in the lumbar region and saline (or air) loss-of-resistance should be used.
- Epidural fat accounts for up to 40 % of volume in lumbar region–sequesters lipophilic local anaesthetic (LA) and limits spread.
Batson Valveless Venous Plexus
- Communicates pelvic, azygos and cranial venous systems.
- Veins engorge in pregnancy, obesity, coughing → higher chance of intravascular catheter or bloody tap.
Skin-to-epidural Depth (adult means)
- Cervical 5–6 cm | Mid-thoracic 3.2 cm | Low-thoracic 4 cm | Lumbar 4.5 cm.
- Depth shortens ~1 cm for every 5 BMI units lost; ultrasound predicts depth to ±5 mm.
Dura & CSF Termination
- Conus medullaris ends around L1 (T12–L3 range).
- Dural sac ends S2 (S1–S4). Dural sleeves extend a few millimetres through foramina–site of possible subdural catheter misplacement.
Sympathetic Outflow & Physiological Consequences
- Preganglionic fibres arise T1–L2.
- High thoracic block (T1–T5) ↓ cardiac sympathetic tone → mild bradycardia & afterload reduction.
- Mid-thoracic block (T5–T10) dilates splanchnic capacitance vessels–be ready with fluid titration ± norepinephrine 0.02–0.04 µg kg⁻¹ min⁻¹.
- Blockade improves gut perfusion & motility, reducing postoperative ileus after abdominal surgery.
Drug Spread Determinants (practical rules)
- Volume dominates–~1 mL plain LA per dermatome in adults (0.7 mL if pregnant).
- Fractionated boluses or PIEB provide wider, more uniform spread than a slow continuous infusion.
- Warmed solutions and steep Trendelenburg slightly increase cephalad spread.
- Catheter advanced 4 cm then withdrawn 1 cm minimises unilateral block and intravascular entry.
Catheter Behaviour
- If threaded >5 cm beyond needle tip: 20 % migrate cephalad, 50 % caudad, 30 % lateral.
- Secure at skin, document mark; reassess each shift to detect migration.
Differential Nerve Susceptibility (why Dilute LA works)
- B-fibres (sympathetic) blocked first → early hypotension.
- C & A-δ (pain), then A-β (touch).
- A-α (motor) needs higher concentration–hence 0.1 % ropivacaine in labour gives analgesia with minimal motor weakness.
Benefits & Indications of Epidural Anaesthesia / Analgesia
- Anaesthesia–Lower-limb arthroplasty, pelvic/urological, colorectal, obstetric Caesarean (when spinal contra-indicated), thoracotomy, open aortic surgery, awake lumbar spine surgery.
- Analgesia–Major thoraco-abdominal surgery, rib fractures, enhanced-recovery colorectal or oesophagectomy pathways, labour (T10-L1 then S2–S4), chronic pain therapy.
- Special situations–Myasthenia gravis (avoid relaxants), autonomic hyper-reflexia (partial efficacy), severe COPD (↓ pneumonia), paediatric scoliosis repair (multimodal).
| Domain | Documented benefit (vs GA ± systemic opioids) | Typical evidence base (2018-25 RCT/registry/meta-analysis) | High-yield clinical examples |
|---|---|---|---|
| Analgesia & opioid-sparing | Superior dynamic pain scores; ≥40 % reduction in cumulative morphine equivalent first 48 h | MULTICEN THOR meta-analysis 2024; PROSPECT colorectal guideline 2023 | Thoracotomy, open/laparoscopic colorectal resection, C-section, scoliosis repair |
| Pulmonary | ↓ pneumonia 25–45 %; ↓ re-intubation & ventilation duration by 0.5-1 d | BJA meta-analysis 2024; COPD propensity study 2023 | Thoracotomy, rib-fracture flail chest, upper-abdominal & oesophagectomy, severe COPD undergoing laparotomy |
| Gastro-intestinal | ↓ time to flatus/solid diet by 15-30 h; ↓ post-op ileus risk | ERAS Society colorectal and pancreatic guidelines 2022 | Pancreatectomy, oesophagectomy, colorectal surgery |
| Haemodynamic / stress response | Blunts catecholamine & cortisol surge; improves insulin sensitivity; ↓ tachy-arrhythmias | Cardiac & vascular surgery RCTs 2022–24 | Off-pump CABG (select), major open aortic repair |
| Thrombo-embolism | 20-30 % lower DVT/PE in hip & knee arthroplasty when used with LMWH | American Hip Society registry 2021 | Total hip/knee replacement, acetabular ORIF |
| Cancer-related | Observational signals of ↓ early recurrence and better NK-cell function; data inconclusive | Pooled oncology-anaesthesia analyses 2023 | Radical prostatectomy, colorectal & ovarian debulking–consider as part of multimodal approach |
| Chronic pain prevention | ↓ incidence of persistent post-thoracotomy pain (NNT≈7) | THORPEC trial 2020 | Thoracotomy, mastectomy, C-section with severe pre-labour pain |
| Renal | Maintains renal blood flow; ↓ AKI after open AAA repair | VASC-TEC registry 2022 | Open abdominal aortic aneurysm, renal transplantation |
| Mortality benefit | 30 % lower 30-day mortality in multi-rib-fracture patients receiving continuous TEA | Trauma Quality Programme 2023 | Severe chest-wall trauma, flail chest |
| Enhanced recovery | Accelerates mobilisation, diet resumption, discharge by 0.5-1.5 d in ERAS pathways | ERAS-CRC meta-review 2024 | Colorectal, pancreatic, major gynaecologic oncology |
| Special populations | Myasthenia gravis–avoids relaxants; Spinal-cord injury > T6–attenuates autonomic hyper-reflexia; Labour–gold-standard analgesia with PIEB + PCEA | Condition-specific cohort studies 2019-25 | See narrative below |
Contraindications to Epidural Block
| Absolute | Relative (case-by-case) |
|---|---|
| Patient refusal or sepsis at puncture site | Mild coagulopathy (platelets 80–100 × 10⁹ L⁻¹), dual antiplatelets after risk assessment |
| Untreated systemic infection / bacteraemia | Severe AS/MS, HOCM (fixed CO) |
| Uncorrected hypovolaemia / shock | Spine deformity/previous fusion—consider US guidance |
| Raised ICP with mass lesion | Neurological disease (MS)–inform consent; no proven causation |
| Epidermoid or vascular malformation at intended site | Moderate thrombocytopenia in pregnancy ≥70 × 10⁹ L⁻¹ |
- Follow ASRA–ESRA 2024 antithrombotic intervals for insertion & catheter removal
Epidural Block in Patients Receiving Antithrombotic Therapy
| Medication | Recommendation |
|---|---|
| NSAIDs (aspirin) | No contraindication |
| Clopidogrel | Wait 7 days before epidural placement |
| 5000 U subcutaneous UFH every 12 hours | No contraindication |
| >10,000 U subcutaneous UFH daily | Safety not established |
| Intravenous heparin | Wait at least 60 minutes after instrumentation before administration; consider aPTT and wait 2–4 hours before catheter removal |
| LMWH thromboprophylactic dose | Wait 12 hours before epidural placement |
| LMWH therapeutic dose | Wait 24 hours before epidural placement |
| Warfarin | Wait for INR to normalize before neuraxial block; remove catheter when INR < 1.5 |
Epidural Adjuvants, Block Optimisation & Redosing
Adjuvants in the Epidural Space (adult doses)
| Class / agent | Typical bolus (labour / post-op) | Infusion (mcg mL⁻¹) | Proven benefits | Common adverse effects |
|---|---|---|---|---|
| Opioids (hydrophilic)–morphine | 2–3 mg | 25–50 | 12–24 h analgesia, ↓ LA dose | Pruritus, N/V, late resp-depression |
| Opioids (lipophilic)–fentanyl | 50–100 µg | 2–5 | Rapid onset, synergy with LA, minimal motor block | Pruritus (dose-related) |
| α₂-agonists–clonidine | 75–150 µg OR 1 µg kg⁻¹ | 1–2 | ↑ block by 30 %, opioid-sparing, ↓ shivering, blunts stress catecholamines in thoracotomy | Hypotension, bradycardia, sedation, dry mouth |
| –dexmedetomidine | 0.5 µg kg⁻¹ | 0.4–0.5 | Similar to clonidine but stronger anti-shiver, better patient comfort | More bradycardia; avoid >0.5 µg kg⁻¹ |
| Ketamine (preservative-free) | 0.5 mg kg⁻¹ | — | Supplemental analgesia when opioid contra-indicated; paediatrics | Dysphoria, ↑ ICP (theoretical) |
| Magnesium sulphate | 50 mg | — | Small ↓ opioid need; inconsistent evidence | Flushing, hypotension at high doses |
| Neostigmine | 500–1000 µg | — | Analgesia without resp-depression | N/V, bradycardia–limits use |
| Dexamethasone | 4–8 mg (IV or epidural) | — | 25–40 % longer painless interval in labour; ↓ PONV | Transient hyperglycaemia |
Clinical Pearls
- Combine low-dose clonidine (≤1 µg kg⁻¹) with fentanyl 2 µg mL⁻¹ for thoracic epidurals: prolongs block ~2 h without significant hypotension.
- Dexmedetomidine ≤0.5 µg kg⁻¹ epidural bolus yields equivalent analgesia to fentanyl with less pruritus and nausea.
- Avoid magnesium or ketamine in obstetrics (limited fetal safety data).
Optimising Injection Site & Catheter Placement
- Align with incision dermatomes–catheter tip 1–2 segments cephalad to surgical field gives fastest, densest block at lowest dose.
- Lumbar injection–cranial spread > caudal; expect L5–S1 sparing (large nerve roots) → supplement with caudal bolus if sacral coverage needed.
- Mid-thoracic catheter–4 mL segments⁻¹ of 0.5 % ropivacaine creates a true band block, sparing lumbar sympathetic fibres → less hypotension and urinary retention.
- PIEB vs continuous–programmed intermittent bolus (e.g. 5 mL q30 min) promotes circumferential spread and reduces motor block compared with 6 mL h⁻¹ continuous infusion.
Epidural Delivery Equipment
- Tuohy needle 17–18 G, 9 cm; 15 cm for BMI > 40 kg m⁻². Curved bevel decreases dural puncture risk.
- Catheters–19 G (with 17 G needle) or 20 G (with 18 G); mark at skin, advance 5 cm (thoracic 4-5 cm) into space.
- Wire-reinforced “spring” catheters reduce kinking and intravascular placement; univs multi-port tips show no outcome difference.
Redosing Schedule for Epidural Local Anaesthetics (adults)
| Local anaesthetic | Typical clinical context | Conc-n (%) | Usual top-up volume* | Two-segment regression (min)† | Recommended top-up interval | Key rationale |
|---|---|---|---|---|---|---|
| Chloroprocaine | Short procedures, conversion to surgical block | 3 | 12–15 mL | 45–75 | 45 min | Very rapid offset → best for <60 min cases or urgent C-section conversion. |
| Lidocaine ± epinephrine 5 µg mL⁻¹ | Caesarean, urgent lower-abdominal / laparoscopy | 2 | 5–10 mL | 60–140 | 60 min | Fast re-establishment of dense block; adrenaline prolongs by ~20 %. |
| Bupivacaine (dense surgical) | Major abdominal/thoracic, lower-limb arthroplasty | 0.5 | 5 mL | 120–180 | 120 min | Long duration—but cumulative cardiotoxicity risk: respect interval. |
| Bupivacaine / Ropivacaine (dilute analgesic) | Labour, ERAS thoraco-abdominal analgesia | 0.1 | Loading: 15–20 mL (≈ 15–20 mg) → Maintenance: 5 mL PIEB q 30 min or CEI 6–10 mL h⁻¹ | 180–260 Measured after the final ≥15 mL bolus once any infusion/PIEB is stopped. |
30 min PIEB (or 120 min if manual intermittent) | Maintains sensory block with minimal motor impairment; high-volume loading gives broad spread, low-volume top-ups keep block refreshed |
| Ropivacaine (dense surgical) | Thoracotomy when motor block acceptable | 0.5 | 6–8 mL | 150–200 | 120 min | Less cardiotoxic and less motor block than equi-potent bupivacaine. |
- Volumes refer to incremental bolus through an existing catheter once two-segment regression is confirmed.
- † Regression times are median ranges from prospective studies and guide maximum interval before re-bolus to avoid breakthrough pain.
- ‡ PIEB = Programmed Intermittent Epidural Bolus (e.g. 5 mL every 30 min with patient-controlled 5 mL lock-out 20 min).
Epidural Technique
Patient Positioning
| Sitting position–key advantages | Lateral decubitus–comparative notes |
|---|---|
| • Spine flexion maximised → inter-spinous gap widens, midline easier to find, especially in obesity / scoliosis. • Shorter skin-to-epidural distance (≈ 0.5 cm less at L3–L4). • Faster procedure time and higher first-pass success in parturients (80 % vs 65 %). • Allows gravity drainage for CSF confirmation if ADP occurs. |
• Better haemodynamic stability in hypovolaemic/trauma patients. • Safer for anaesthetised or sedated patients (airway control). • Facilitates ultrasound scanning without probe slipping. |
Identifying the Epidural Space
| Method | Best-evidence practice | Pitfalls / complications |
|---|---|---|
| Loss-of-resistance (LOR) to saline | 10 mL filled syringe; continuous or pulsatile pressure. Saline avoids air-related PDPH and pneumocephalus. | False LOR if needle enters interspinous ligament gap or paraspinous muscle—advance slowly. |
| LOR to air | Acceptable where saline unavailable; keep air ≤1 mL. | ↑ PDPH (RR 1.6), patchy block, nerve-root irritation, venous air embolism. Air LOR is still used but is associated with more patchy blocks and rare pneumocephalus; evidence for higher PDPH rates is mixed, yet most centres now prefer saline. |
| Hanging-drop | Useful in mid-/upper thoracic levels where epidural pressure is sub-atmospheric. | Ineffective lumbar; unreliable in COPD / morbid obesity (pressure less negative). |
| Ultrasound guidance | Pre-puncture scan–marks midline, depth ±5 mm accuracy. Improves first-pass success and reduces ADP by 45 %. | Learning curve; real-time scanning difficult beyond L2 in obesity. |
| Epidural waveform (pulse/respiratory) | Pressure transducer confirms pulsatile-respiratory waveform after catheter placement. | False-positives if catheter tip intrathecal. Requires sterile pressure tubing. |
Midline Gaps & Ligamentum Flavum Variants
- Midline fusion defect present in 10–15 % of adults (up to 25 % of obstetric patients).
- Expect softer ligaments in pregnancy → subtler “pop”.
- Always re-insert stylet before redirecting to prevent tissue coring and occult blockage.
Catheter Handling
- Insertion–advance 4–6 cm in lumbar, 3–4 cm in thoracic space; stop if paraesthesia or blood/CSF.
- Fixation–secure at skin mark = (needle depth + catheter length inside). Cover with occlusive sterile dressing and bacterial filter; document depth.
- Test dose–3 mL lidocaine 1.5 % + adrenaline 5 µg mL⁻¹; HR rise ≥20 min⁻¹ → intravascular, motor block → intrathecal.
- Labour analgesia:
- Maintenance–continuous infusion 5–8 mL h⁻¹ or programmed intermittent bolus 5 mL q30 min with PCEA (greater spread, less motor block).
- 0.1 % ropivacaine + fentanyl 2 µg mL⁻¹, 5 mL PIEB q30 min with PCEA backup
- 0.1 % ropivacaine + sufentanil 0.5 µg mL⁻¹, 5–10 mL PIEB at 30to 50-min intervals
- Lower concentrations (0.0625–0.08 %) have also been investigated, but the “5 mL q30 min” regimen you quoted is drawn from the literature that standardised on 0.1 %.
- Maintenance–continuous infusion 5–8 mL h⁻¹ or programmed intermittent bolus 5 mL q30 min with PCEA (greater spread, less motor block).
Alternative Needle Approaches
- Paramedian
- Entry 1 cm lateral & 1 cm inferior to lower edge of superior spinous process, 15° medial–cephalad.
- Ideal for calcified midline ligaments, kyphoscoliosis, mid-thoracic epidurals.
- Ultrasound visualises lamina and ligamentum flavum “target sign” for precise angulation.
- Taylor (modified paramedian L5–S1)
- Entry 1 cm medial & 1 cm caudal to PSIS, 45–55° medial-cephalad trajectory.
- Useful when sitting impossible (hip fracture traction) or previous lumbar fusion hardware.
- Walk needle off sacrum if bone encountered then advance into ligamentum flavum.
Complications Specific to Technique
| Issue | Incidence / risk factors | Prevention |
|---|---|---|
| Accidental dural puncture | 0.5–2 % (lumbar); shallow depth (<4 cm) triples risk | Slow advancement, use saline LOR, stop at first CSF flash, ultrasound depth prediction |
| Patchy / unilateral block | Catheter orifice within epidural fat septum, <3 cm insertion, midline plica | Insert ≥4 cm, pull back 1 cm if patchy, use intermittent bolus |
| Intravascular placement | 5–15 % with single-shot; higher in pregnancy | Aspirate, epinephrine test dose, wire-reinforced catheter if high risk |
| Epidural vein cannulation using air LOR | More common with >1 mL air | Prefer saline; minimal air if used |
Initiation & Management of an Epidural Block
Confirming Catheter Position
- Pharmacological test dose (adult)–3 mL lidocaine 1.5 % + epinephrine 5 µg mL⁻¹ (total 15 µg).
- Intrathecal → rapid dense motor block (<3 min) or unexpected sensory level.
- Intravascular → HR ↑ ≥ 20 % or SBP ↑ ≥ 15 % within 60 s (less reliable in β-blocked or labouring patients).
- In obstetrics and paediatrics consider fractionated 1 mL test doses to limit sympathetic response.
- Repeat position checks before every bolus/top-up: negative aspiration, no neuro-sensory change after 1 mL saline flush.
Dosing Strategy
| Block level | Loading rule-of-thumb | Example (70 kg) | Typical maintenance* |
|---|---|---|---|
| Lumbar | 1–2 mL segment⁻¹ of chosen LA | L2–S1 (6 seg) → 10 mL bupivacaine 0.25 % | Continuous 0.1 % bupivacaine 6–8 mL h⁻¹ ± fentanyl 2 µg mL⁻¹ |
| Mid-thoracic | 0.7 mL segment⁻¹ | T6–T10 (5 seg) → 4 mL ropivacaine 0.5 % | 0.2 % ropivacaine 5 mL h⁻¹ ± hydromorphone 5 µg mL⁻¹ |
| Caudal | 3 mL segment⁻¹ | S2–L2 (5 seg) → 15 mL ropivacaine 0.25 % | Paediatric continuous caudal 0.1 % ropi 0.2 mL kg⁻¹ h⁻¹ |
- Programmed-intermittent epidural bolus (PIEB) 5 mL q30 min with patient-controlled epidural analgesia (PCEA) lock-out 20 min provides wider spread and lower hourly LA consumption than continuous infusion.
Top-up (redosing)–integrate Pharmacokinetics
- Use the unified table supplied earlier: redose when the sensory block has regressed two dermatomes or at the time-to-top-up limit, whichever occurs first.
Problem-solving during Placement
| Problem (encounter) | Likely cause | Evidence-based fix |
|---|---|---|
| Needle “flops”; deviates laterally | Off mid-line entry | Re-palpate spinous processes; reposition 0.5 cm cephalad |
| Bone ≤ 2 cm depth | Spinous process | Insert at caudal edge of interspace; flex spine further |
| Bone ≥ 4 cm depth | Lamina (too lateral) | Withdraw to skin; re-angle 10° medial |
| Constant bony feel | Ossified ligaments, scoliosis | Switch to paramedian or Taylor approach; ultrasound scout |
| No catheter advance / false LOR | Needle tip in fat, midline gap | Inject 3 mL saline to predistend; advance needle 1–2 mm |
| Blood return | Epidural vein | Pull back 1 cm; if persistent, new level or lateral position |
| Warm clear fluid | Dural puncture | Offer continuous spinal OR re-site ≥ one space above |
| Paresthesia on threading | Catheter hitting nerve root | Withdraw to leave 3 -4 cm in space; if persistent, re-site |
| Spinous processes impalpable | Morbid obesity | Sitting position + ultrasound depth & mid-line mark |
| Inability to flex spine | Ankylosis, instrumentation | Paramedian or Taylor; lateral flexed position |
Catheter Management Pearls
- Insertion depth–4–6 cm lumbar, 3–4 cm thoracic; deeper increases unilateral block/intravascular risk.
- Wire-reinforced catheters decrease vein cannulation and kinking–preferred for prolonged infusions (>48 h).
- Apply bacterial filter; document depth/mark; reassess at every shift
- Remove catheter when coagulation parameters meet ASRA–ESRA time intervals.
Avoiding Epidural-vein Cannulation
- Lateral over sitting in pregnancy/obesity.
- Use flexible or wire-reinforced catheter.
- Insert ≤ 6 cm; stop if blood appears in Tuohy hub before threading.
- Pre-distend space with 3–5 mL saline via needle before catheter insertion.
Relative Order of Peak Plasma Concentration of Local Anesthetic Associated with Regional Anesthesia (Descending Order)
| Regional Anesthesia Site |
|---|
| Intercostal |
| Caudal |
| Paracervical |
| Epidural |
| Brachial plexus |
| Sciatic/femoral |
Spinal Block Failure
Summary
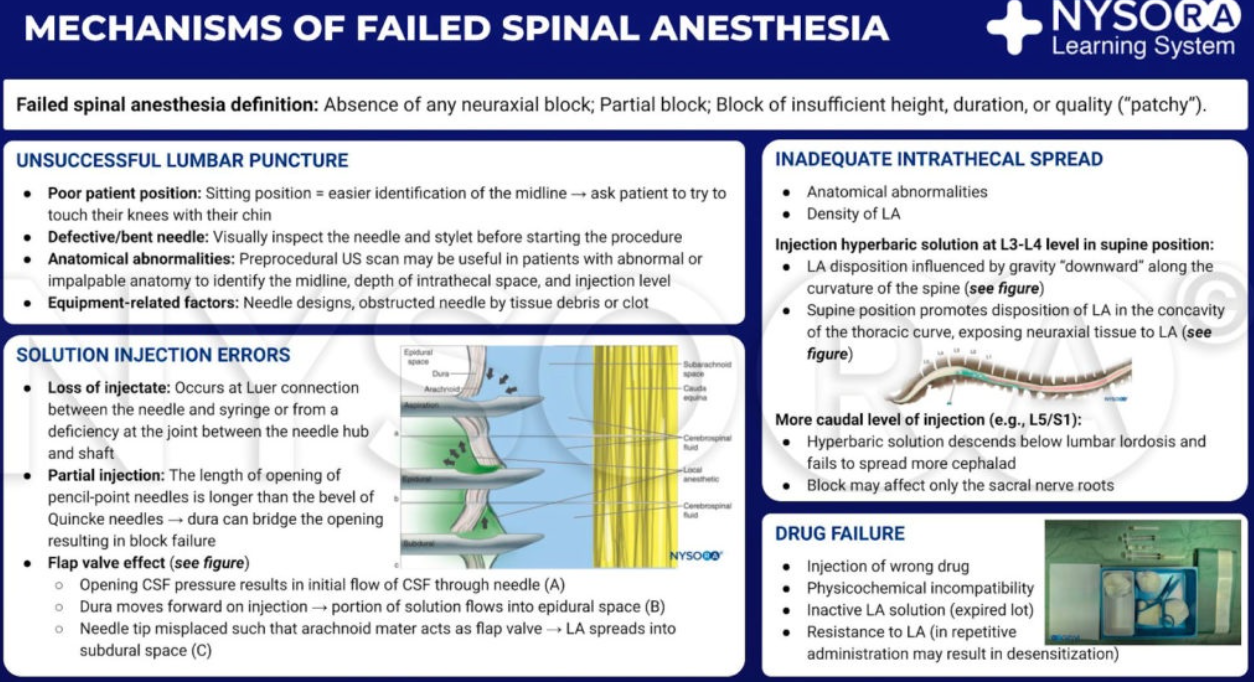
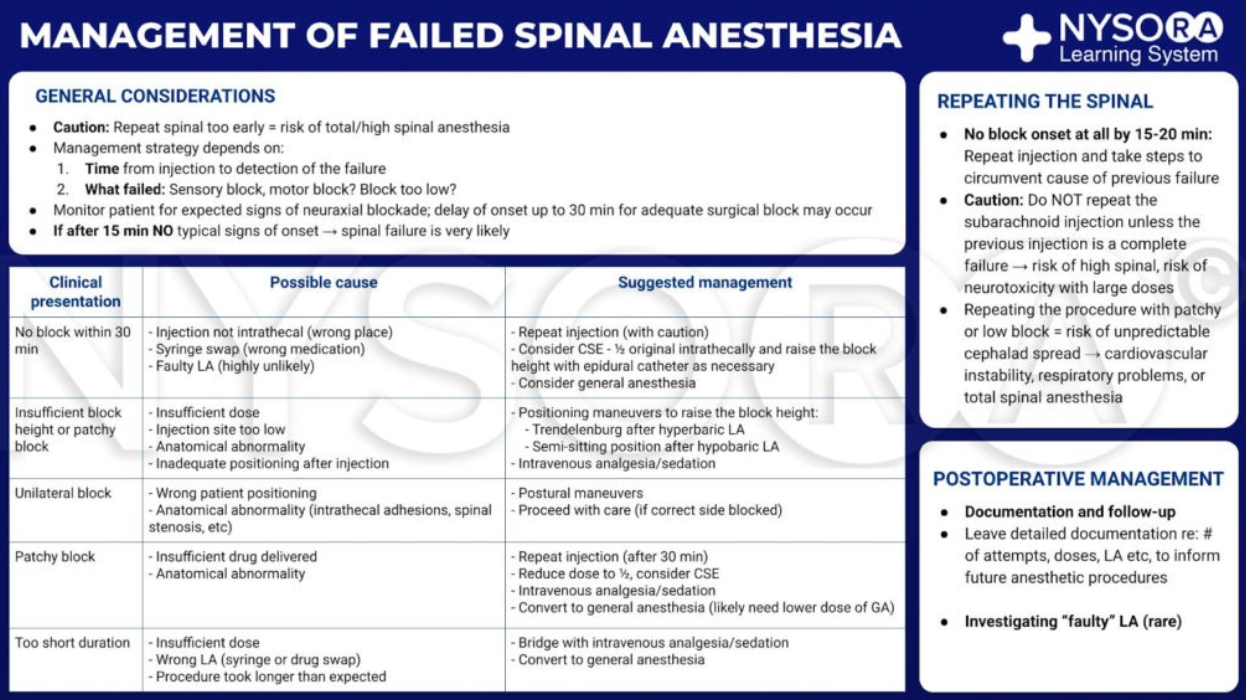
Definition & Clinical Impact
- Total failure–complete absence of sensory or motor change by 15 min after intrathecal injection.
- Partial failure–block present but inadequate in height, density (pain on incision) or duration for the planned surgery.
- Modern database studies put the overall failure rate at 4–7 %; conversion to general anaesthesia (GA) doubles PONV and prolongs discharge, so anticipation and early rescue are essential
Five-phase Framework for Root-cause Analysis
| Phase | Key success factors | Typical error → consequence |
|---|---|---|
| 1 Lumbar puncture | Correct mid-line / paramedian trajectory; free CSF flow | Missed dural puncture → no block |
| 2 Drug injection | Entire dose delivered intrathecally; slow, steady (<0.3 mL s⁻¹) | Epidural/subdural injection → patchy or unilateral block |
| 3 CSF spread | Baricity matched to patient position; appropriate volume | Inadequate height (low dose, head-up tilt, large CSF volume) |
| 4 Neural action | Potent, active LA; correct dose for duration | Out-of-date / ester LA degradation → short block |
| 5 Patient management | Position maintained 3–5 min; haemodynamics supported | Hypotension → spinal cord ischaemia, high block |
Principal Aetiologies of Failure
| Group | Salient points & preventive tips |
|---|---|
| A. Technical access problems | • Obesity, scoliosis, ankylosis: use pre-puncture ultrasound marking or paramedian/Taylor approach. • Mid-line ligamentum flavum gaps (10–15 %): advance slowly; feel for double “pops”. |
| B. Pseudo-CSF aspiration | Epidural LA “reservoir” or Tarlov cyst fluid may mimic CSF; confirm by free flow + swirling after aspiration rather than glucose dip-stick. |
| C. Dose selection / delivery error | Syringe swap; partial loss of injectate; dose below ED95 (e.g., <8 mg bupivacaine for hip fracture). Mitigate: label syringes, aspirate 0.5 mL CSF preand post-injection. |
| D. Unpredictable intrathecal spread | Plain (isobaric) solutions more variable; hyperbaric under gravity is most reliable. Trendelenburg 10° for under-height block within first 10 min. |
| E. Inactive or degraded LA | Rare with amides; avoid multi-use vials >30 days; discard any solution discoloured or beyond expiry. |
| F. True LA resistance | Extremely rare (channelopathy); consider after ≥2 class-different failures; plan GA. |
Structured Block Assessment (within 5–10 min)
- Motor–ask patient to lift straight leg (L2-S1).
- Sensory–light touch then cold from sacrum cephalad; aim ≥2 dermatomes above incision.
- Quality–apply gentle forceps pinch in surgical field before prep to detect patchiness.
- If sensory level has not advanced for 3 min, predict failure and initiate rescue plan rather than “wait and hope”.
Rescue Algorithms
| Presentation (≤ 15 min) | Likely cause | Safe rescue |
|---|---|---|
| No block | Wrong space / inactive LA | Repeat spinal with new kit & drug, different interspace; or proceed GA. |
| Low block height | Dose loss, high CSF volume | Trendelenburg (hyperbaric); epidural top-up if catheter present; IV ketamine 0.25 mg kg⁻¹ for incision. |
| Unilateral block | Patient tilt, dural fold | Turn patient towards unblocked side + hip flexion; if unchanged, consider epidural supplementation or GA. |
| Patchy block | Subdural injection | Evaluate haemodynamics; convert to GA (repeat intrathecal hazardous). |
| Early regression (<60 min) | Under-dosing | Epidural or IV analgesia; GA if surgery ongoing. |
- Never re-inject intrathecally until ≥20 min have elapsed and cause is corrected–risk of total spinal.
Practical Prevention Bundle
| Step | Evidence-based intervention |
|---|---|
| Pre-puncture | Ultrasound depth & mid-line marking in obesity/geriatric spine. |
| During puncture | 25 G pencil-point needle, stylet re-inserted for redirects. |
| Pre-injection | Verbally double-check drug, dose & baricity; aspirate free-flow CSF. |
| Injection | Give full dose over ≥15 s; keep bevel orientation constant. |
| Position | Maintain intended tilt 3–5 min; then surgeon may position. |
| Monitoring | NIBP q1 min first 5 min; treat hypotension early with NE infusion. |
Key Take-home Messages
- 80 % of failures stem from technical or dose errors–checklist discipline and ultrasound lower risk
- Hyperbaric bupivacaine 10–12 mg remains most reliable single-shot for adult lower-limb/abdominal surgery; reduce to 7-9 mg in obstetrics or ≥70 yrs.
- Aim to detect inadequacy by 10 min; decisive early rescue (epidural, IV adjunct, or GA) avoids patient harm and theatre delay.
Links
Past Exam Questions
Post-Partum Headache and Post-Dural Puncture Headache (PDPH)
a) List 4 causes of a post-partum headache, other than a dural puncture. (2)
b) How could you as the anaesthetist reduce the risk of post-dural puncture headache (PDPH)? (4)
c) List pharmacological alternatives to an epidural blood patch that are effective for treating PDPH. (4)
References:
- NYSORA. (n.d.). Spinal Anesthesia. Retrieved from https://www.nysora.com/techniques/neuraxial-and-perineuraxial-techniques/spinal-anesthesia-2/
- NYSORA. (n.d.). Epidural Anesthesia & Analgesia. Retrieved from https://www.nysora.com/techniques/neuraxial-and-perineuraxial-techniques/epidural-anesthesia-analgesia/
- Bao, N., Shi, K., Wu, Y. et al. Dexmedetomidine prolongs the duration of local anesthetics when used as an adjuvant through both perineural and systemic mechanisms: a prospective randomized double-blinded trial. BMC Anesthesiol 22, 176 (2022).(https://doi.org/10.1186/s12871-022-01716-3)
- Talke P, Anderson BJ. Pharmacokinetics and pharmacodynamics of dexmedetomidine-induced vasoconstriction in healthy volunteers. Br J Clin Pharmacol. 2018 Jun;84(6):1364-1372. doi: 10.1111/bcp.13571. Epub 2018 Apr 2. PMID: 29495085; PMCID: PMC5980451.(https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.13571)
- Sween LK, Xu S, Li C, O’Donoghue MA, Ciampa EJ, Kowalczyk JJ, Li Y, Hess PE. Low-dose intravenous dexmedetomidine reduces shivering following cesarean delivery: a randomized controlled trial. Int J Obstet Anesth. 2021 Feb;45:49-55. doi: 10.1016/j.ijoa.2020.11.004. Epub 2020 Nov 17. PMID: 33293185. (https://pubmed.ncbi.nlm.nih.gov/33293185/)
- Kang H, Lim T, Lee HJ, Kim TW, Kim W, Chang HW. Comparison of the effect of dexmedetomidine and midazolam under spinal anesthesia for cesarean delivery: a randomized controlled trial, single center study in South Korea. Anesth Pain Med (Seoul). 2023 Apr;18(2):159-168. doi: 10.17085/apm.22257. Epub 2023 Apr 28. PMID: 37183284; PMCID: PMC10183612 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10183612/).
- Liu S, Zhao P, Cui Y, Lu C, Ji M, Liu W, Jiang W, Zhu Z, Sun Q. Effect of 5-μg Dose of Dexmedetomidine in Combination With Intrathecal Bupivacaine on Spinal Anesthesia:(https://pubmed.ncbi.nlm.nih.gov/32222361/)
- Khosravi F, Sharifi M, Jarineshin H. Comparative Study of Fentanyl vs Dexmedetomidine as Adjuvants to Intrathecal Bupivacaine in Cesarean Section: A Randomized, Double-Blind Clinical Trial. J Pain Res. 2020 Oct 7;13:2475-2482. doi: 10.2147/JPR.S265161. PMID: 33116789; PMCID: PMC7548853.(https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7548853/#:~:text=In%20the%20present%20study%20intrathecal,compared%20to%2025%20%CE%BCg%20fentanyl.)
- NYSORA. (n.d.). Neuraxial Anesthesia and Peripheral Nerve Blocks in Patients on Anticoagulants. Retrieved from https://www.nysora.com/topics/foundations-of-regional-anesthesia/patient-management/neuraxial-anesthesia-peripheral-nerve-blocks-patients-anticoagulants/
- Neuman MD, Markowitz AJ, Sieber FE, et al.; REGAIN Investigators and the Canadian Perioperative Anesthesia Clinical Trials Group. Spinal anesthesia or general anesthesia for hip surgery in older adults. N Engl J Med. 2021;385(22):2025-2035
- Li T, Jia Z, Ni J, et al. Effect of regional vs general anesthesia on postoperative delirium in older patients undergoing hip fracture surgery: the RAGA randomized clinical trial. JAMA. 2022;327(1):50-59.
- Rodgers A, Walker N, Schug SA, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anesthesia: results from overview of randomized trials. BMJ. 2000;321(7275):1493.
- NYSORA. (n.d.). Mechanisms and Management of Failed Spinal Anesthesia. Retrieved from https://www.nysora.com/techniques/neuraxial-and-perineuraxial-techniques/mechanisms-management-failed-spinal-anesthesia/
- Image: Novice Anaesthesia. (2021). Infographics. Retrieved April 24, 2025, from https://www.gasnovice.com/infographics
- Cook TM, Counsell D, Wildsmith JA. Major complications of central neuraxial block: report on the Third National Audit Project. Br J Anaesth. 2009;102(2):179-90.
- Kopp SL, Horlocker TT, Warner ME, et al. Regional anesthesia in the patient receiving antithrombotic therapy: ASRA 2023 guidelines (5th edition). Reg Anesth Pain Med. 2025;50(1):1-29.
- Choi PT, Galinski SE, Takeuchi L, et al. PDPH is a common complication of neuraxial blockade in parturients: a meta-analysis of obstetrical studies. Can J Anaesth. 2003;50(5):460-9.
- Hart JR, Whitacre RJ. Pencil-point needle in prevention of post-spinal headache. JAMA. 1951;147(7):657-8.
- Obasuyi BI, Fyneface-Ogan S, Mato CN. Post-dural puncture headache in obstetrics: a review. Afr Health Sci. 2015;15(2):540-7.
- Roofthooft E. Low-dose spinal anaesthesia for Caesarean section to prevent spinal-induced hypotension. Curr Opin Anaesthesiol. 2016;29(3):268-71.
- Ngan Kee WD, Lee SW, Ng FF, et al. Randomized double-blind comparison of norepinephrine and phenylephrine for maintaining blood pressure during spinal anesthesia for Cesarean delivery. Anesthesiology. 2015;122(4):736-45.
- Heesen M, et al. Vasopressors for the management of hypotension after spinal anesthesia for elective cesarean section: a systematic review and network meta-analysis. Int J Obstet Anesth. 2019;37:143-55.
- Mohta M, et al. Efficacy of prophylactic norepinephrine infusion for prevention of postspinal hypotension in cesarean section: A meta-analysis. Anaesthesia. 2019;74(11):1540-50.
- Qi X, et al. Efficacy and safety of intrathecal dexmedetomidine as adjuvant to bupivacaine: a meta-analysis of RCTs. Clin Drug Investig. 2016;36(5):383-95.
- Gao L, et al. Intrathecal dexmedetomidine vs. fentanyl as adjuvants for cesarean section analgesia: A meta-analysis. BMC Anesthesiol. 2020;20(1):81.
- Van de Velde M, et al. Pain relief and epidural neural blockade in obstetrics. In: Chestnut’s Obstetric Anesthesia, 6th ed. 2020. p. 541-80.
- Bollag L, et al. Ultrasound-assisted epidural placement in obesity: a randomized controlled trial. Anesthesiology. 2019;131(6):1131-8.
- Sprung J, et al. Outcomes after regional versus general anesthesia for abdominal aortic aneurysm repair. Anesthesiology. 2009;111(5):980-7.
- Rodgers A, et al. Reduction in postoperative mortality and morbidity with epidural or spinal anesthesia: Results from overview of randomised trials. BMJ. 2000;321(7275):1493.
- Hollmann MW, et al. 2-Chloroprocaine: old drug, new tricks. Curr Opin Anaesthesiol. 2018;31(5):608-13.
- Bulger EM, et al. Epidural analgesia improves outcome after multiple rib fractures. Surgery. 2004;136(2):426-30.
- Myles PS, et al. Influence of anaesthetic techniques on outcome in oncological surgery: a meta-analysis. Anaesthesia. 2016;71(4):344-55.
- Svircevic V, et al. Thoracic epidural anesthesia for cardiac surgery: a randomized trial. Anesthesiology. 2011;114(2):262-70.
- Kinsella SM, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anesthesia. Anaesthesia. 2018;73(1):71-92.
- Van den Berg AA, et al. Incidence and causes of failed spinal anaesthesia: 10-year observational study. Br J Anaesth 2022;129:624-32.
Summaries:
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “1f5d1705-befe-4be4-bd98-a82e8db21e2d”



