{}
Introduction
GasNovice Labour Analgesia Recipes
GasNovice Labour Analgesia Recipes 2
Pre-Labour Assessment
| Task |
Details |
| History & examination |
Obstetric, medical, anaesthetic history; airway, spine, CVS, respiratory review |
| Laboratory tests |
Platelet count only if indicated (PET, HELLP, anticoagulants)–neuraxial acceptable if ≥ 70 × 10⁹ L⁻¹ and stable |
| Fetal status |
Confirm reassuring CTG with obstetrician |
| Resources |
Check trained staff, resuscitation and difficult-airway equipment |
| Consent |
Technique, benefits, risks (PDPH, motor block, conversion to CS), financial implications |
Pain Pathways in Labour
| Stage |
Pain type |
Dermatomes |
Afferent pathway |
| 1st (latent/active) |
Visceral (uterine contraction, cervical dilation) |
T11–L1 → T10–L1 |
Sympathetic fibres via uterovaginal → inferior hypogastric plexus → dorsal roots |
| 2nd |
Somatic (perineum, vagina, pelvic floor) |
T10–S4 (pudendal S2-S4) |
Pudendal & perineal branches via sacral plexus |
Analgesic Options
Non-pharmacological (evidence-based)
- Continuous one-to-one support, breathing & relaxation techniques
- Water immersion (significant pain-score reduction)
- Acupuncture, acupressure, sterile-water injections
- TENS–modest benefit in back-dominant pain
Systemic Pharmacological Agents
| Drug |
Typical regimen |
Key points |
| Pethidine (meperidine) |
25–50 mg IM or 10–25 mg IV (max 100 mg/4 h) |
Limited by nausea & neonatal sedation; avoid <4 h before delivery |
| Remifentanil PCA |
20–40 µg demand bolus, 2 min lock-out; no basal |
Rapid onset/offset; superior satisfaction vs pethidine; requires 1:1 monitoring & pulse oximetry |
| Fentanyl bolus |
25–100 µg IV q1 h PRN |
Minimal neonatal depression; useful while awaiting neuraxial |
| Ketamine |
10–15 mg IV for rescue just before delivery |
>1 mg kg⁻¹ may cause uterine hypertonus |
| Antiemetics/Anxiolytics |
Promethazine 12.5 mg IV; hydroxyzine 25 mg IM |
Use sparingly; routine midazolam discouraged (amnestic, neonatal hypotonia) |
| NSAIDs |
Avoid during labour–risk of ductus arteriosus constriction & uterine atony |
|
Neuraxial Analgesia
General Principles
- Low-dose LA (≤0.1 % bupivacaine/ropivacaine) + lipophilic opioid (fentanyl 2 µg mL⁻¹ or sufentanil 0.3 µg mL⁻¹) balances analgesia and motor preservation.
- Programmed Intermittent Epidural BolusPIEB (6–10 mL q30–45 min) with PCEA demand 5–8 mL achieves lowest rescue rate and drug consumption.
- Vasopressor (phenylephrine 25–50 µg min⁻¹) superior to fluid loading for hypotension prophylaxis.
Fluid & Vasopressor Strategy
| Strategy |
Evidence |
| Crystalloid co-load 10–15 mL kg⁻¹ at block placement |
Non-inferior to colloid when combined with prophylactic vasopressor |
| Colloid preload |
Marginal additional benefit but ↑ cost and allergy risk |
Epidural Technique
- Placement–L3-4 or L4-5, loss-of-resistance to saline; limit air ≤2 mL.
- Catheter–Multi-orifice 4–5 cm in space.
- Test–Incremental 3 mL 0.1 % bupivacaine + adrenaline 5 µg mL⁻¹ (observe HR & sensory change).
- Initial bolus–10 mL 0.1 % bupivacaine + fentanyl 2 µg mL⁻¹ in 5 mL aliquots.
- Maintenance–PIEB 8 mL q40 min with PCEA 6 mL, lock-out 10 min; no basal infusion.
- Troubleshooting–Additional 5 mL bolus; adjust catheter; re-site early if asymmetric or ineffective after two clinician boluses.
Conversion to Caesarean Anaesthesia
- Treat epidural placed > 60 min ago as untested.
- Top-up with alkalinised lidocaine 2 % with adrenaline + fentanyl (total 15–20 mL).
- If surgical block not to T4 within 10 min → spinal (preferred) or GA.
Dosage Summary at GSH
- Test Dose:
- 2.5 mL of 2% lignocaine, wait 5 min
- Bolus Dose:
- Mix: 5 mL 0.5% bupivacaine + 4 mL saline + 50 mcg fentanyl
- 10ml= 0.25% Bupivacaine and 5ug/ml fentanyl
- Administer two 4 mL boluses 3 min apart
- Measure BP every 5 min, test level in 20 min (Aim T8-T10)
- Infusion:
- Mix: 76 mL saline + 20 mL 0.5% bupivacaine + 200 mcg fentanyl (0.1% bupivacaine with 2ug:ml fentanyl in total 100ml)
- Rate: 8-14 mL/hour (start when block no higher than T8)
- Breakthrough Pain:
- 1.5 mL per segment of 0.25% bupivacaine or 5-10 mL 0.1% solution with 25 mcg fentanyl
- Top-Up for C/S:
- Mix: 17 mL 2% lignocaine + 50 mcg fentanyl + 1 mL 8.4% sodium bicarbonate + 1 mL 1/10000 adrenaline
- Administer 5 mL boluses after test dose
- Requires 16-22 mL for effective T4 block
Advantages of Regional over Systemic Analgesia
- Neonatal–Higher umbilical pH, less need for resuscitation than systemic opioids.
- Maternal–Reduced pulmonary aspiration risk, lower catecholamines → improved uteroplacental perfusion, readiness for emergency CS, superior postpartum pain control with intrathecal morphine.
Motor Block Classification
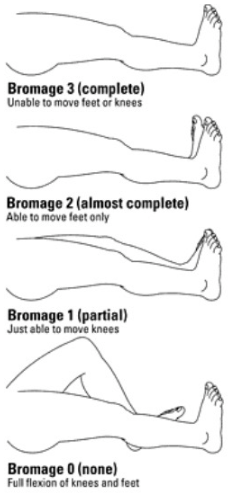
Spinal/Epidural Techniques
Local Anaesthetics (low-concentration regimens) for Epidural
| Drug |
Common concentration range* |
Typical hourly consumption† |
| Bupivacaine |
0.05 – 0.10 % |
6–12 mg h⁻¹ |
| Levobupivacaine |
0.05 – 0.10 % |
6–12 mg h⁻¹ |
| Ropivacaine |
0.08 – 0.20 % |
8–16 mg h⁻¹ |
- *Concentrations ≤0.1 % minimise motor block and instrumental delivery rates without compromising analgesia.
- †Assumes average basal or programmed dosing; adjust for maternal weight and technique.
Opioid and Non-opioid Adjuncts
| Agent (typical epidural concentration) |
Benefits |
Caveats |
| Opioid Adjuncts |
– |
– |
| Fentanyl 1.5–3 µg mL⁻¹ |
Rapid onset, improves sacral analgesia, reduces LA requirement |
Pruritus, nausea, respiratory depression (rare) |
| Sufentanil 0.3–0.5 µg mL⁻¹ |
High potency, long duration, synergistic with LA |
Similar side effects as fentanyl; avoid overdose |
| Morphine (preservative-free) 0.1–0.2 mg for spinal (bolus) |
Long-duration analgesia post-C/S |
Delayed respiratory depression; avoid in labour epidural |
| Non-opioid Adjuncts |
– |
– |
| Epinephrine 2 µg mL⁻¹ |
↓ Minimum Local Analgesic Concentration (MLAC); prolongs block |
↑ Motor block; tachycardia |
| Clonidine 1–2 µg mL⁻¹ |
Potentiates LA without ↑ motor block |
Maternal sedation, hypotension; FDA boxed warning (US) |
| Dexmedetomidine 0.3–0.5 µg mL⁻¹ |
Superior early analgesia, LA-sparing; may enable opioid-free regimens |
Dose-dependent bradycardia; limited obstetric data |
| Neostigmine 2–4 µg mL⁻¹ |
Only useful when combined with clonidine |
Nausea at higher doses |
| Routine magnesium or ketamine adjuvants remain investigational. |
|
|
- Opioid sparing with dexmedetomidine or esketamine reduces pruritus and preserves uterine activity, but experience remains limited to small trials.
Opioid-Only Neuraxial Analgesia (Labour)
- Description: Administration of lipophilic opioids (e.g., fentanyl, sufentanil) or hydrophilic opioids (e.g., morphine) into the intrathecal or epidural space without local anaesthetic.
- Use Case: Often used for rapid-onset analgesia when delivery is imminent or in cases where LA-induced motor block is undesirable.
- Common Regimens:
- Intrathecal:
- Fentanyl 10–25 µg or
- Sufentanil 2.5–5 µg
- Epidural:
- Fentanyl 50–100 µg bolus or
- Sufentanil 10–20 µg bolus
- Advantages: No motor block, fast onset, improved maternal mobility.
- Limitations: Short duration (especially with intrathecal lipophilic opioids), pruritus, nausea, and rare respiratory depression.
- Not recommended as sole maintenance for prolonged labour—best for short duration or bridge therapy.
Epidural Test Dose–2023 Consensus Update
| Position |
Rationale & current practice |
| For an adrenaline-containing test dose (3 mL lignocaine 1.5 % + adrenaline 5 µg mL⁻¹) when imminent conversion to CS is likely. |
Facilitates early recognition of intravascular or intrathecal catheters. |
| Against universal test dosing in low-dose labour regimens; instead favour aspiration + incremental 5 mL dosing. |
Low LA concentrations carry minimal toxicity; tachycardic response blunted in pregnancy; false negatives common. |
Epidural Maintenance
| Technique |
Key points |
Evidence-based advantages |
Important limitations |
| Manual intermittent bolus |
10–15 mL clinician-delivered bolus when pain recurs |
Simple equipment |
Breakthrough pain, variable spread, high workload |
| Continuous epidural infusion (CEI) |
6–12 mL h⁻¹ of dilute LA ± opioid |
Steady analgesia in busy units |
Higher total LA dose than bolus-based regimens |
| Patient-controlled epidural analgesia (PCEA) |
Demand bolus 5–8 mL, lock-out 10–15 min |
↓ LA use; ↑ maternal satisfaction |
Requires patient engagement; risk of under-dosing in late labour |
| PCEA + basal infusion |
Basal 4–6 mL h⁻¹ plus PCEA |
Fewer clinician interventions; superior comfort in long second stage |
LA consumption slightly ↑ vs demand-only PCEA |
| Programmed intermittent epidural bolus (PIEB) |
Automated 6-10 mL bolus q30-60 min (often with PCEA) |
Superior dermatomal spread, less motor block, ↓ rescue dosing, ↑ satisfaction |
Requires modern pumps; optimal volume–interval pairing still under study |
| Computer-integrated PCEA (CIPCEA) |
Pump adjusts basal rate to recent PCEA requests |
Maintains low LA use while preventing breakthrough pain |
Cost; limited availability |
| Closed-loop/automated systems |
Algorithm titrates bolus size to real-time pain scores or PCEA frequency |
Promising pilot data; potential to reduce clinician workload |
Experimental |
- Current best practice
- PIEB combined with PCEA is the preferred technique in high-resource settings, delivering the lowest rescue bolus rate and drug consumption while maintaining maternal satisfaction. In low-resource units, CEI with dilute solutions remains acceptable when pump technology is limited.
Impact of Neuraxial Analgesia on Labour & Delivery Outcomes
Duration of Labour
| Stage |
Contemporary evidence |
Practical implications |
| First stage |
Meta-analyses of >25 000 parturients show no clinically relevant prolongation (mean difference <10 min) when low-concentration epidurals (≤0.1 % bupivacaine-equivalent) are used. |
Timing of epidural (early vs ≥5 cm dilatation) does not influence progress or mode of delivery. |
| Second stage |
Pooled data indicate a modest increase (≈15–20 min) that is greatest in nulliparas and with higher LA concentrations; ultra-low solutions (≤0.06 %) abolish this effect. |
Do not discontinue the epidural—reduce basal rate once the head is on the perineum to preserve pushing sensation. |
| Third stage |
No prolongation attributable to epidural analgesia. |
|
Caesarean Section (CS) Rate
- Large RCTs and recent population analyses confirm no increase in overall CS rate with neuraxial versus systemic opioid analgesia, irrespective of early initiation.
- Observational work of >500 000 births shows neuraxial analgesia is associated with a 35 % reduction in severe maternal morbidity (SMM), reinforcing its safety profile.
Instrumental Vaginal Delivery
- High-dose epidurals (>0.1 % bupivacaine-equivalent) increase assisted vaginal delivery (AVD).
- Network meta-analysis demonstrates that low/ultra-low LA or PIEB + PCEA regimens restore AVD rates to baseline.
- Encourage upright positioning and coached pushing to mitigate residual risk.
Fetal & Neonatal Outcomes
| Variable |
Finding |
Evidence |
| Fetal heart rate (FHR) changes |
Transient decelerations occur in up to 15 % after rapid-onset CSE, attributed to acute ↓ maternal catecholamines. |
Usually resolve within 10 min; continuous CTG for 30 min post-dose. |
| Severe neonatal morbidity |
No increase in 5-min Apgar < 7 or NICU admission with modern regimens. |
|
| Breast-feeding |
Low-dose LA ± fentanyl epidurals do not impair initiation or duration of breast-feeding. |
|
Technique Comparison
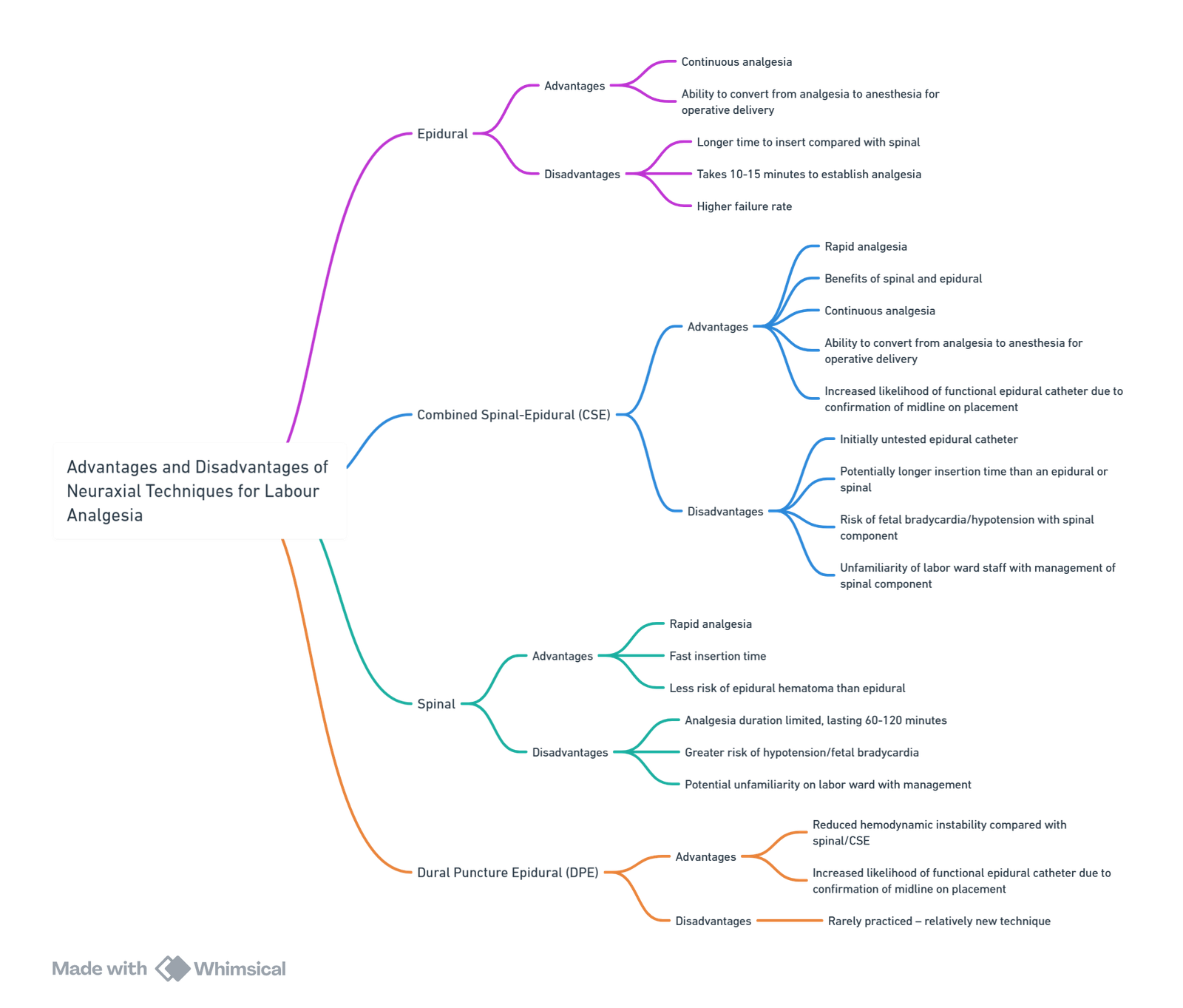
Combined Spinal–Epidural (CSE) & Dural-Puncture Epidural (DPE)
| Aspect |
CSE |
DPE |
| Onset of analgesia |
2-5 min (opioid ± 5 mg bupivacaine) |
6-8 min, faster than standard epidural |
| Fetal effects |
Higher incidence of transient bradycardia (up to 11 %). |
Similar to epidural |
| Block quality/failure |
Lowest failure rate; ideal for rapid progress |
↓ unilateral & motor block compared with epidural. |
| Limitations |
Can’t test epidural for 60-90 min; ↑ PDPH risk |
Still investigational; requires pencil-point spinal needle |
Continuous Caudal Analgesia
- Rarely employed owing to
- Higher infection and misplacement risk, especially in advanced labour; documented rectal or foetal injections.
- Large volumes (≥20 mL) needed for low-thoracic spread → ↑ maternal plasma LA concentrations.
Remains a niche option (e.g., severe lumbar scoliosis/fusion).
Selecting the Optimal Technique
| Technique |
Preparation time |
Analgesia quality & onset |
Motor block |
Conversion to CS anaesthesia |
| Epidural (PIEB ± PCEA) |
Moderate |
Excellent; titratable |
Minimal with ≤0.1 % LA |
May require 2-chloroprocaine top-up |
| CSE |
Fast |
Rapid & dense |
Minimal (opioid-only SA) |
Reliable with epidural top-up |
| DPE |
Moderate |
Near-CSE quality |
Minimal |
Similar to epidural |
| Continuous caudal |
Slow |
Variable |
Minimal |
Limited by cephalad spread |
- Key take-away: Low-concentration LA techniques (PIEB + PCEA or DPE) maximise maternal satisfaction and safety while keeping instrumental and CS rates at baseline.
Complications
| Complication |
Updated incidence/notes |
Prevention & management pearls |
| Hypotension after epidural initiation |
≈ 10–15 % with low-dose solutions |
Treat with 250 mL crystalloid coload and phenylephrine 50–100 µg IV; exclude intrathecal placement |
| Pruritus |
Up to 30 % with lipophilic opioids |
Ondansetron 4 mg IV or low-dose nalbuphine 2 mg IV effective; dexmedetomidine adjunct reduces incidence |
| Maternal fever |
15–25 % after ≥4 h epidural; inflammatory rather than infectious |
Paracetamol and active maternal cooling; antibiotics only if sepsis suspected |
| Shivering |
Common with neuraxial block |
Low-dose pethidine (meperidine) 12.5 mg IV or forced-air warming |
| Inadequate analgesia / catheter failure |
6–12 % with ultrasound-guided placement; lower with combined spinal-epidural (CSE) |
Re-site early; use ultrasound to confirm midline; consider DPE or CSE in difficult anatomy |
| Local anaesthetic systemic toxicity (LAST) |
Inadvertent IV injection ≈ 1: 5000 |
Immediate 20 % lipid emulsion 1.5 mL kg⁻¹ bolus → 15 mL kg⁻¹ h⁻¹ infusion |
| Post-dural puncture headache (PDPH) |
35–50 % after recognised Dural puncture using Tuohy needle |
Early mobilisation acceptable; offer sphenopalatine ganglion block; epidural blood patch (EBP) 15–20 mL if debilitating |
| High or total spinal |
1: 1400–16000 |
Call for help; left uterine displacement; vasopressors; airway control with ketamine 1.5 mg kg⁻¹ and suxamethonium 1 mg kg⁻¹ if apnoeic |
| Persistent motor block / prolonged neural deficit |
Rare; rule out epidural haematoma (MRI <6 h) |
Stop infusion, urgent imaging, neurosurgical referral |
High (Total) Spinal Neuraxial Block
Definition & Incidence
- High spinal: Sensory block spreads above T3, producing clinically significant cardio-respiratory compromise.
- Total spinal: Unintentional large intrathecal dose produces brain-stem anaesthesia with apnoea and profound hypotension.
- Contemporary obstetric data (89 726 neuraxial cases, 2021-2023) estimate an incidence of 1: 3 100 for high/total spinal; 15 % require tracheal intubation.
Risk Factors
- Rapid or excessive intrathecal injection (e.g. epidural “top-up” inadvertently intrathecal).
- Short stature (<150 cm), obesity, late pregnancy.
- Supine or head-down positioning before block has fixed.
- Multi-orifice epidural catheter misplaced in subarachnoid, subdural or intradural space.
- Recent large epidural bolus (<30 min) followed by spinal or CSE.
Recognition (progressive timeline)
| Time course |
Respiratory signs |
Cardiovascular signs |
Conscious level |
| Early (T4–T2) |
Dyspnoea, weak cough, RR 12–15 min⁻¹, SpO₂ ≥ 95 % |
SBP ↓ 10–20 %, HR normal |
Anxious but alert |
| Impending (C7–C5) |
Hypoventilation, unable to speak, SpO₂ 90–95 % |
SBP ↓ > 20 %, ± HR < 60 min⁻¹ |
Dizzy, paraesthesiae upper limbs |
| Established (C4 and above) |
Apnoea |
Severe hypotension ± bradycardia/asystole |
Loss of consciousness |
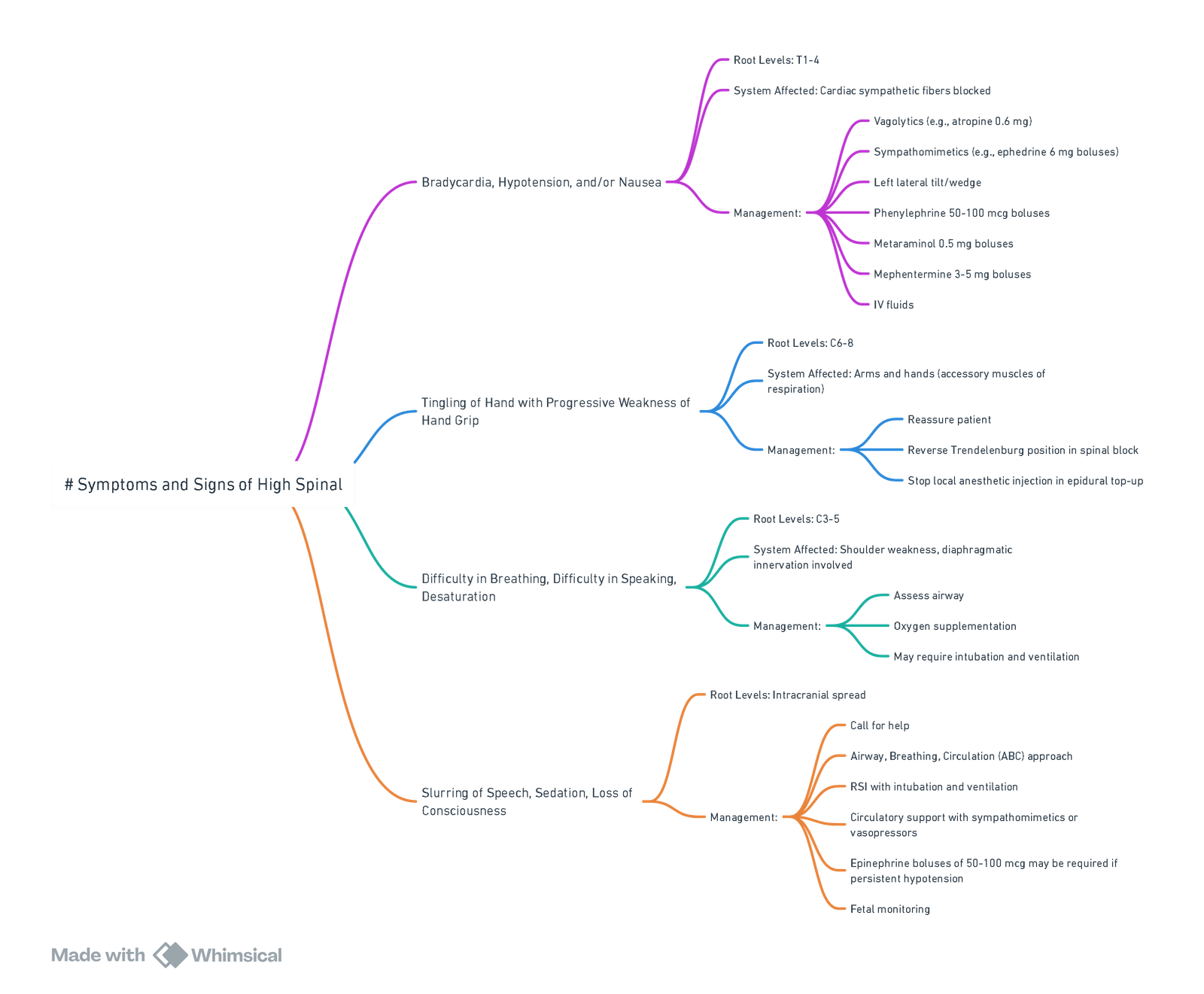
View or edit this diagram in Whimsical.
Management Algorithm
- Call for help, tilt uterus left 15°.
- 100 % oxygen; support ventilation with bag-mask ± cricoid pressure.
- Secure airway if apnoeic/obtunded: ketamine 0.5 mg kg⁻¹ IV + suxamethonium 1 mg kg⁻¹ (avoid propofol/thiopental).
- Circulation
- Ephedrine 6–12 mg IV if HR < 60 min⁻¹.
- Phenylephrine 100 µg IV if HR ≥ 60 min⁻¹ and SBP < 80 % baseline.
- Adrenaline 10–20 µg IV for refractory hypotension or arrest; start infusion 0.05–0.1 µg kg⁻¹ min⁻¹ as needed.
- Position head-up once haemodynamically stable to limit further cephalad spread.
- Post-event imaging (MRI) if persistent neurological deficit > 6 h to exclude cord compression.
Post-Dural Puncture Headache (PDPH)
Background
- Definition: Orthostatic headache within 5 days of a recognised or occult dural puncture.
- Incidence: ~35 % after unintentional dural puncture (UDP) with a 16–18 G Tuohy; <1 % after pencil-point spinal.
Risk Factors
- Female sex; > age < 50 y; large cutting needle; bevel perpendicular to dura; multiple attempts; failure to replace stylet.
Presentation
- Onset: Typically develops 12-48 hours after dural puncture (up to 5 days).
- Duration: Usually resolves spontaneously within 1-2 weeks.
- Characteristics: Positional headache (worse when upright, better when supine), bilateral, often frontal or occipital.
- Associated Symptoms: Neck stiffness, photophobia, nausea, subjective hearing changes (tinnitus, hypoacusia).
Pathophysiology
- Mechanism: Thought to be due to CSF leakage through the dural hole, causing CSF or intracranial hypotension.
Differential Diagnosis
- Important to rule out other more sinister causes of headache.
- Indications for Urgent Imaging: CT/MRI, neuro consult if diagnosis is unclear, presence of fever/chills, neurological signs, seizures, decreased level of consciousness (LOC), or if two epidural blood patches (EBPs) are ineffective.
Management
Conservative
- Daily review until resolution.
- Bed rest for temporary relief.
- Maintain hydration.
- Thromboprophylaxis considering the timing if a blood patch is used.
- Simple analgesia.
- Stronger opioids (<72 hours) can be offered.
- Caffeine (200-300 mg doses, total 900 mg/24 hours) for short-term benefit.
- Stool softeners to avoid straining.
Epidural Blood Patch (EBP)
- Indications: If conservative measures fail or the headache is debilitating.
- Effectiveness: 65-98% effective after one treatment; can repeat if necessary.
- Contraindications: Same as those for neuraxial anesthetic techniques.
- Procedure:
- Administer at the level or one level below the previous entry point.
- Use 20 mL of blood, stopping if discomfort occurs.
- No need for concomitant antibiotics.
- Bed rest for 2 hours post-procedure.
- Ongoing monitoring and review 4 hours after the procedure.
Other Options
- Trans-nasal sphenopalatine ganglion blocck (0.5 mL 2 % lignocaine per nostril).
- Greater occipital nerve block for refractory occipital pain.
- Imaging / neurology consult: Thunderclap onset, focal neurology, seizure, fever, or failure of two EBPs.
Prevention of PDPH
- Insufficient evidence for clear guidance.
- Consider injecting ~10 mL of preservative-free normal saline into the subarachnoid space if UDP occurs.
- Consider placing an intrathecal catheter for 24 hours, ensuring medications are not inadvertently injected into the catheter.
Spinal Hypotension
- SBP < 90 mmHg or > 2030 % fall from baseline (same for MAP).
Prevention Bundle (level A evidence)
| Component |
Details |
| Left uterine displacement |
≥15° from time of spinal |
| Crystalloid coload |
10–15 mL kg⁻¹ |
| Phenylephrine infusion |
Start 25–50 µg min⁻¹ immediately after intrathecal injection; titrate q1 min to keep SBP ≥ 90 % baseline |
| Alternative first-line |
Norepinephrine 2–3 µg min⁻¹ ↓ bradycardia, ↑ CO (network meta-analysis 2025). |
Rescue Algorithm
- SBP 80–90 % baseline, HR ≥ 60 min⁻¹: Increase infusion or give phenylephrine 100 µg IV.
- SBP < 80 % baseline + HR < 60 min⁻¹: Ephedrine 5–10 mg IV ± atropine 0.6 mg IV.
- Refractory / HR < 50 min⁻¹: Adrenaline 10 µg IV bolus → infusion 0.05–0.2 µg kg⁻¹ min⁻¹.
- Resource-limited settings: intermittent boluses—phenylephrine 50–100 µg, ephedrine 10 mg, metaraminol 0.5 mg.
| Tool |
Variables |
Notes |
| PRAM score |
Age > 25 y, HR > 90 min⁻¹, MAP < 90 mmHg (0–3) |
Score = 3 doubles hypotension risk; limited external validation. |
| Shock index ≥ 0.9 |
HR/SBP |
Baseline SI ≥ 0.9 independently predicts post-spinal hypotension. |
Regional for C/S
Single-Shot Spinal Anaesthesia
| Component |
Contemporary recommendations |
Evidence |
| LA dose |
Hyperbaric bupivacaine 0.5 % 9–12 mg (ED95 ≈ 11 mg; consider 8–9 mg in <150 cm stature) |
Dose-finding RCTs 2023–24 |
| Opioid adjuncts |
Fentanyl 15–25 µg or sufentanil 5–10 µg → faster onset, denser block, no neonatal depression |
Large meta-analysis 2022 |
| Intrathecal morphine |
100 µg (range 75–150 µg) prolongs analgesia to 18–24 h; monitor ≥ 12 h for delayed respiratory depression |
ASA adjuvant statement 2024 |
| Obesity considerations |
110 mm (4.3 in) 25 G pencil-point needle suffices for most. Use 120–150 mm needle or pre-procedural ultrasound if BMI ≥ 40 kg m⁻². |
RCTs 2023–25 |
- Hypotension prophylaxis
Phenylephrine infusion 25–50 µg min⁻¹ from time of intrathecal injection halves the incidence of pre-delivery hypotension compared with boluses
Epidural Anaesthesia (Extension of Labour Epidural)
| Urgency category |
Suggested top-up |
Onset (T4) |
Notes |
| Elective / category III |
Lidocaine 2 % + adrenaline 5 µg mL⁻¹ + sodium bicarbonate 1 mEq per 10 mL + fentanyl 100 µg; total 15–20 mL in 5 mL increments |
5–7 min |
Alkalinisation accelerates onset |
| Urgent / category II |
Chloroprocaine 3 % 15–20 mL |
2–3 min |
Rapid metabolism; minimal neonatal exposure |
| Category I (immediate threat) |
Consider spinal (8–10 mg bupivacaine + opioid) or GA if conversion uncertain |
|
|
Best practice
- Aspirate + incremental dosing remain safer than routine test-dose; adrenaline test-dose reserved when surgical anaesthesia is imminent.
- Treat inadequate block early: additional 5 mL alkalinised lidocaine; if still patchy, proceed to CSE or GA.
Alternative Regional Techniques
| Technique |
Typical use |
Key limitations |
| Paracervical block |
Adjunct for first-stage pain when neuraxial unavailable |
Risk of fetal bradycardia; seldom sole technique |
| Pudendal block |
Perineal analgesia for instrumental/second stage |
Haematoma, infection, pudendal neuropathy |
| Continuous caudal |
Rare—spinal abnormalities |
Higher infection & misplacement rates; large LA volumes required |
“Walking” (Ambulatory) Epidural / CSE
- Low-dose CSE (e.g. bupivacaine 2.5 mg + fentanyl 25 µg intrathecal, followed by PIEB + PCEA ≤0.06 % bupivacaine) preserves motor power and allows ambulation when strict criteria are met. UK safety checklist (Hywel Dda UHB 2024) requires:
- Reassuring CTG, engaged presenting part
- Stable vitals (< 10 % change)
- Modified Bromage 0; able to straight-leg raise and step unassisted
- Continuous support & 15-min intermittent CTG monitoring
- Adherence to such protocols maintains mobility without increasing falls or instrumental delivery rates
Epidural Analgesia in VBAC
| Concern |
Evidence-based answer |
| Masks uterine rupture pain |
Break-through pain (“epidural sieve”) combined with CTG changes detects rupture earlier than pain alone. Large cohort (> 1 500 VBACs) shows no delay in diagnosis. |
| Attenuates haemodynamic response to bleeding |
Sympathectomy effect small; vigilance for unexplained hypotension still required |
| Safety |
No increase in uterine rupture or neonatal morbidity; facilitates rapid conversion to surgical anaesthesia if needed |
Troubleshooting an Epidural During Labour
- Rule out non-neuraxial pain (bladder, cervical laceration, uterine rupture).
- Check catheter position → re-site early if uncertain.
- Top-up options
- Dilute bolus 5–10 mL 0.1 % bupivacaine + 2 µg mL⁻¹ fentanyl.
- For asymmetric block: place less-blocked side dependent, give incremental boluses.
- Persistent failure or > 2 clinician boluses → re-site or convert to CSE.
Extending Epidural Labour Analgesia to Caesarean Anaesthesia
| Step |
Practical tip |
| Assess time since last dose |
Treat as no block if > 60 min or multiple rescues required. |
| Plan drug & volume |
Aim 15–20 mL to reach T4. Use alkalinised lidocaine or chloroprocaine for speed. |
| Monitor |
Continuous BP, HR, SpO₂; phenylephrine infusion ready. |
| Failure to achieve dense block within 10 min |
Perform spinal (preferred) or GA; do not delay for repeated epidural boluses. |
Enhanced Recovery After Caesarean (ERAC)–2025 Update
- Why ERAC? Standardised, evidence-based care bundles shorten length of stay by ~12 h, halve moderate–severe pain scores, and reduce opioid use by 40 %.
Pre-operative Phase
| Core elements (ERAS Society 2022) |
Key details |
| Patient education & expectations |
Written & video resources; discuss early mobilisation, feeding, analgesia |
| Limit fasting |
Solids ≤ 6 h; clear carbohydrate drink (50 g) 2 h pre-op |
| Haemoglobin optimisation |
Treat iron-deficiency anaemia ≥2 weeks pre-delivery; aim Hb ≥ 110 g L⁻¹ |
| Breast-feeding preparation |
Lactation counselling; skin-to-skin within 5 min of delivery |
- Optional enhancements–smoking cessation, anxiety screening, birth companion training.
Intra-operative Phase
| Core elements (Part 2 guideline) |
Practical implementation |
| Prevent spinal hypotension |
Phenylephrine 25–50 µg min⁻¹ (or norepinephrine 2–3 µg min⁻¹ if maternal HR < 60) from block placement |
| Normothermia |
Forced-air warming + IV fluids warmed to 37 °C |
| Antibiotic prophylaxis |
Cefazolin 2 g ≥ 30 min pre-incision (add metronidazole if prolonged rupture) |
| Nausea / vomiting (I- & PONV) |
Ondansetron 4 mg IV + dexamethasone 8 mg IV |
| Multimodal analgesia start |
Spinal morphine 100 µg; IV paracetamol 1 g; consider TAP block if spinal opioid omitted |
| Optimal uterotonics |
Oxytocin 1–3 IU slow IV then infusion 7.5 IU h⁻¹; avoid ergometrine in hypertensive women |
| Immediate bonding |
Skin-to-skin while on table; delayed cord clamping ≥ 60 s |
- Recommended extras–goal-directed fluid therapy (2 mL kg⁻¹ h⁻¹ maintenance), cell-saving if anticipated blood loss > 1 L, liberal clear-fluid intake in theatre.
Post-operative Phase
| Core elements (Post-op guideline 2025) |
Targets |
| Early oral intake |
Sips in PACU; full diet within 4 h |
| Early mobilisation |
Sit up by 4 h; ambulate ≥10 m by 8 h |
| Urinary catheter removal |
≤ 8 h if ambulating and no magnesium infusion |
| Multimodal analgesia |
Regular paracetamol + NSAID (if normotensive & no PPH); breakthrough oxycodone 2–5 mg PO |
| VTE prophylaxis |
LMWH 40 mg SC from 6 h & anti-embolism stockings |
| Anaemia remediation |
IV iron if Hb < 100 g L⁻¹ 24 h post-op |
| Breast-feeding support |
Dedicated nurse within first hour |
| Discharge |
Criteria-led; goal ≤ 30 h post-op |
- Recommended extras–glycaemic control < 8 mmol L⁻¹, chewing gum to hasten bowel function.
Neuraxial Analgesia & Anaesthesia in Pre-eclampsia (PET)
| Consideration |
Current best evidence |
| Platelet threshold |
Safe to proceed if stable count ≥ 70 × 10⁹ L⁻¹; verify within 6 h in HELLP. |
| Labour epidural |
Blunts pain-induced hypertension and catecholamine surges; facilitates rapid conversion to anaesthesia if urgent CS required. |
| Spinal / CSE |
Lower incidence of severe hypotension in PET, but maintain phenylephrine (or NE) infusion readiness. |
| Vasopressor of choice |
Phenylephrine first-line; norepinephrine offers less bradycardia and higher cardiac output. |
| Fluids |
Restrict crystalloids; treat hypotension primarily with vasopressors (no autoregulation of uteroplacental flow). |
| Airway |
Anticipate oedema–ramped position, 6 cm ETT, THRIVE/NODESAT for apnoeic oxygenation. |
| Drugs to avoid |
Ergometrine; rapid IV oxytocin bolus (>3 IU); NSAIDs if renal or platelet dysfunction present. |
| Post-op |
Higher PPH and pulmonary oedema risk–HDU/ICU observation for 24 h. |
Dexmedetomidine as an Adjuvant in Neuraxial and Peripheral Regional Anesthesia: Updated Evidence and Guidelines (2020–2025)
Mechanism of Action
- α2-Adrenergic Agonism: Dexmedetomidine (DEX) exhibits high selectivity for α2 over α1 receptors (α2:α1 ≈ 8–10:1), leading to sedation, analgesia, and sympatholysis.
- Hyperpolarization via HCN Channel Blockade: DEX inhibits hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, enhancing analgesia and potentiating local anesthetic effects.
- Analgesia: Mediated through inhibition of substance P and glutamate release in the dorsal horn of the spinal cord.
- Sedation: Achieved by activation of α2 receptors in the locus coeruleus
- Vasoconstriction: Mild α1 activity may delay systemic uptake of local anesthetics, prolonging their effect.
General Safety
- Regulatory Status: DEX is not FDA-approved for obstetric use or non-intravenous routes.
- Pregnancy Category: Classified as Category C; crosses the placenta but results in minimal fetal exposure.
- Neonatal Outcomes: Studies report no significant changes in fetal heart rate, Apgar scores, or neonatal outcomes.
- Neurotoxicity: No reported neurotoxic effects with neuraxial or perineural administration
Clinical Applications and Dosing by Route
1. Intravenous (IV) Use in Obstetric Anesthesia
- Indications: Intraoperative sedation, analgesia, shivering control, and potential PTSD prevention.
- Dosing:
- Sedation: Continuous infusion at 0.2–0.5 µg/kg/hr.
- Shivering: Single bolus of 0.5 µg/kg.
- Benefits:
- Minimal respiratory depression.
- Reduced intraoperative pain in cesarean section patients.
- Effective shivering control compared to alternatives like meperidine.
- Potential reduction in PTSD incidence post-cesarean section.
2. Intrathecal Dexmedetomidine
- Proposed Mixture:
- DEX: 2–4 µg (based on 4 µg/mL preservative-free vial).
- Local Anesthetic: 10–13.5 mg of 0.75% hyperbaric bupivacaine.
- Optional: Intrathecal morphine for extended analgesia.
- Outcomes:
- Faster onset and prolonged duration of sensory and motor block.
- Reduced postoperative pain and shiveriring
- Decreased need for intraoperative opioids.
- Potential for hypotension and bradycardia; preparedness with vasopressors and anticholinergics recommended.
- Evidence
- A study determined the ED50 of intrathecal DEX as an adjuvant to plain ropivacaine for spinal anesthesia during cesarean section to be approximately 5.9 µg with 8 mg ropivacaine and 3.1 µg with 10 mg ropivacaine.
3. Epidural Dexmedetomidine
A. Epidural Conversion for Cesarean Section
- Mixture: 4 µg DEX added to 10–20 mL of 2% lidocaine, with or without bicarbonate.
- Outcome: Faster onset and improved intraoperative analgesia.
B. Epidural Top-Ups (Second Stage Labor)
- Mixture: 2–4 µg DEX added to standard bolus (e.g., 4 mL of 0.1% ropivacaine with fentanyl or 2–3 mL of 0.2% ropivacaine).
- Benefit: Enhanced assessment of block effectiveness and improved analgesia.
C. Continuous Labor Epidural Infusions
- Mixture: 0.1% ropivacaine with 0.3–0.5 µg/mL DEX.
- Outcomes:
- Reduced local anesthetic consumption.
- Decreased patient-controlled epidural analgesia (PCEA) boluses.
- Lower incidence of pruritus and nausea.
- No significant maternal or neonatal side effects.
- Optimal concentration identified as 0.3 µg/mL to minimize motor block.
- Evidence:
- A study found that dexmedetomidine combined with local anesthetic for epidural labor analgesia improved pain scores without prolonging labor stages
4. Peripheral Nerve Blocks
- Mixtures:
- Outpatient Procedures: 0.5–1 µg/kg DEX with local anesthetic.
- Inpatient Procedures: 1–2 µg/kg DEX with local anesthetic.
- Benefits:
- Shortened onset time of anesthesia.
- Prolonged analgesia by approximately 3–4 hours.
- Opioid-sparing effect.
- Cautions:
- Transient hypotension observed at higher doses (1.5–2 µg/kg).
- Contraindicated in patients with bradycardia, hypotension, pulmonary hypertension, obstructive sleep apnea, frailty, or shock.
- Key Studies
- A meta-analysis revealed that perineural DEX doses of 30–50 µg are appropriate, providing prolonged analgesia without increasing the risk of bradycardia and hypotension
5. Nebulized Dexmedetomidine for Post-Dural Puncture Headache (PDPH)
- Dose: 1 µg/kg diluted to 4 mL with saline, administered every 12 hours.
- Benefits:
- Reduction in cerebral blood flow, alleviating PDPH symptoms.
- Increased cerebrospinal fluid (CSF) pressure due to decreased absorption.
- Non-invasive and opioid-sparing approach.
- Minimal hemodynamic or sedative effects.
Dexmedetomidine as an Adjuvant in Regional Anesthesia
| Route / Application |
Dose / Concentration |
Evidence Summary |
Benefits |
Comparisons / Notes |
| Intrathecal (CS, ortho) |
2–4 µg+ 10–13.5 mg 0.75% bupivacaine ± morphine |
Liu et al. 2020; Mo et al. 2023: prolonged block (~2h), reduced pain, ED50 ≈ 3.1–5.9 µg |
Fast onset, dense and prolonged block, ↓ shivering, ↓ opioid need |
Superior to fentanyl for block duration and shivering reduction |
| Epidural Conversion (CS) |
4 µg + 10–20 mL 2% lidocaine ± bicarbonate |
Riham et al. 2016: improved onset, analgesia vs epinephrine |
Rapid CS conversion, better intra-op analgesia |
Comparable or better than epinephrine |
| Epidural Top-Up (2nd Stage) |
2–4 µg + 4 mL 0.1% ropivacaine ± fentanyl OR 2–3 mL 0.2% ropivacaine |
Qian et al. 2021; Yang et al. 2020: faster onset, less nausea/shivering vs fentanyl |
Improved block efficacy, less shivering and nausea |
Faster onset and fewer side effects than fentanyl |
| Continuous Epidural Infusion |
0.3–0.5 µg/mL in 0.1% ropivacaine |
Wei et al. 2022; Pang et al. 2022: fewer PCEA boluses, lower LA use, optimal dose = 0.3 µg/mL |
Longer duration, fewer PCEA uses, less pruritus |
Better tolerability than sufentanil |
| Peripheral NB (Outpatient) |
0.5–1 µg/kg + local anesthetic |
Dai et al. 2018: prolonged block (~3–4h), better analgesia |
Faster onset, prolonged analgesia, opioid-sparing |
↑ bradycardia/hypotension vs ropivacaine alone |
| Peripheral NB (Inpatient) |
1–2 µg/kg + local anesthetic |
Packiasabapathy et al. 2017; Jung et al. 2018: 2 µg/kg superior to 1 µg/kg for duration (~20h) |
Longest block duration (~20h), ↓ postop opioid use |
2 µg/kg better than 1 µg/kg; ↑ hypotension risk |
| Nebulized (PDPH) |
1 µg/kg diluted to 4 mL saline, q12h |
Kumar et al. 2019; Mowafy et al. 2021: ↓ PDPH severity, increased CSF pressure, minimal side effects |
Non-invasive PDPH relief, minimal sedation |
Alternative to conservative PDPH management |
Links
Past Exam Questions
Safety and Efficacy of Labour Analgesia Modalities
Critically evaluate the safety and efficacy of the following modalities for labour analgesia.
a) Remifentanil patient-controlled analgesia (PCA) pump. (5)
b) Single-shot spinal anaesthetic. (5)
Pain Pathways in Labour and Locoregional Techniques for Perineal Repair
a) Define the stages of labour and describe the transmission of nociceptive stimuli to the spinal cord (“pain pathways”) in each phase of labour as well as during caesarean section. (8)
b) A patient has a third-degree tear after vaginal delivery. List two locoregional techniques that may be used to facilitate a repair. (2)
Risks of Epidural Analgesia in Labour
You are obtaining consent for epidural analgesia from a parturient in labour.
Define and quantify 5 of the most pertinent risks of this procedure. (10)
Labour Epidural Consent and Risks
a) In consenting a patient for a labour epidural, how would you quantify the risks for the following complications:
- i) Temporary nerve damage. (1)
- ii) Permanent nerve damage. (1)
- iii) Dural puncture. (1)
- iv) Failure. (1)
b) List three precautions that you would take to mitigate the risk of catheter migration. (3)
c) A patient experiences pain during the second stage of labour despite a well-functioning epidural in the first stage. Give two reasons for this. (3)
References:
- Allman K, Wilson I, O’Donnell A. Oxford Handbook of Anaesthesia. Vol. 4. Great Clarendon Street, Oxford, OX2 6DP, United Kingdom: Oxford University Press; 2016. 1295 p. Allman et al. – Oxford Handbook of Anaesthesia.pdf
- Butterworth J, Mackey D, Wasnick J. Morgan and Mikhail’s Clinical Anesthesiology, 7th Edition. 7th edition. New York: McGraw Hill Medical; 2022.
- Volmanen, Petria; Palomäki, Outib; Ahonen, Jounic. Alternatives to neuraxial analgesia for labor. Current Opinion in Anaesthesiology 24(3):p 235-241, June 2011. | DOI: 10.1097/ACO.0b013e328345ad18
- South African Medical Journal 2017;107(12):1127-1131. DOI:10.7196/SAMJ.2017.v107i12.123901. The Calgary Guide to Understanding Disease. (2024). Retrieved June 5, 2024, from https://calgaryguide.ucalgary.ca/
- Bower, J. and Kinsella, S. (2020). Preventing and treating hypotension during spinal anaesthesia for caesarean section. BJA Education, 20(11), 360-361. https://doi.org/10.1016/j.bjae.2020.08.001
- Bao, N., Shi, K., Wu, Y. et al. Dexmedetomidine prolongs the duration of local anesthetics when used as an adjuvant through both perineural and systemic mechanisms: a prospective randomized double-blinded trial. BMC Anesthesiol 22, 176 (2022).(https://doi.org/10.1186/s12871-022-01716-3)
- Talke P, Anderson BJ. Pharmacokinetics and pharmacodynamics of dexmedetomidine-induced vasoconstriction in healthy volunteers. Br J Clin Pharmacol. 2018 Jun;84(6):1364-1372. doi: 10.1111/bcp.13571. Epub 2018 Apr 2. PMID: 29495085; PMCID: PMC5980451.(https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.13571)
- Singh S, Kannan SK, et al. Programmed intermittent epidural bolus for labour analgesia: systematic review and network meta-analysis. Br J Anaesth. 2023;131:240-54. pubmed.ncbi.nlm.nih.gov
- Barber KM, et al. Influence of PIEB dosing variables on labour analgesia outcomes: meta-analysis. Curr Opin Anaesthesiol. 2023;36:400-8. sciencedirect.com
- Chen C, et al. Norepinephrine versus phenylephrine to prevent hypotension after spinal anaesthesia for caesarean section: systematic review and meta-analysis. Int J Obstet Anesth. 2024;52:103-12. pmc.ncbi.nlm.nih.gov
- Liu X, et al. Vasopressor management of spinal anaesthesia-induced hypotension in obstetrics: review. Anesth Analg. 2023;136:1078-90. [pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC11089295/?utm_source=chatgpt.com
- Khan M, et al. Comparison of phenylephrine and norepinephrine for post-spinal hypotension: meta-analysis. Anaesthesia. 2023;78:1065-73. pmc.ncbi.nlm.nih.gov
- Kinsella SM, et al. Consensus statement on the management of hypotension during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73:71-92. researchgate.net
- American Society of Anesthesiologists. Statement on quality metrics for obstetric anaesthesia. ASA; 2023. asahq.org
- Uppal V, et al. Evidence-based clinical practice guidelines on post-dural puncture headache: multisociety consensus. Anaesthesia. 2023;78:1613-29. pubmed.ncbi.nlm.nih.gov
- Nazir N, et al. Trans-nasal sphenopalatine ganglion block for post-dural puncture headache: systematic review and meta-analysis. Br J Anaesth. 2023;131:1182-92. pubmed.ncbi.nlm.nih.gov
- Lou S, et al. ED90 of epidural esketamine with 0.075 % ropivacaine for labour analgesia. Front Pharmacol. 2023;14:1169415. frontiersin.org
- Brown CA, et al. Modern labour epidural analgesia and labour outcomes. Am J Obstet Gynecol. 2023;229:678-85. sciencedirect.com
- Heesen M, Stewart A, Fernando R. Use of vasopressors for maternal hypotension: past, present and future. Anaesthesia. 2024;79:20-30.
- Sween LK, Xu S, Li C, O’Donoghue MA, Ciampa EJ, Kowalczyk JJ, Li Y, Hess PE. Low-dose intravenous dexmedetomidine reduces shivering following cesarean delivery: a randomized controlled trial. Int J Obstet Anesth. 2021 Feb;45:49-55. doi: 10.1016/j.ijoa.2020.11.004. Epub 2020 Nov 17. PMID: 33293185. (https://pubmed.ncbi.nlm.nih.gov/33293185/)
- Kang H, Lim T, Lee HJ, Kim TW, Kim W, Chang HW. Comparison of the effect of dexmedetomidine and midazolam under spinal anesthesia for cesarean delivery: a randomized controlled trial, single center study in South Korea. Anesth Pain Med (Seoul). 2023 Apr;18(2):159-168. doi: 10.17085/apm.22257. Epub 2023 Apr 28. PMID: 37183284; PMCID: PMC10183612 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10183612/).
- Liu S, Zhao P, Cui Y, Lu C, Ji M, Liu W, Jiang W, Zhu Z, Sun Q. Effect of 5-μg Dose of Dexmedetomidine in Combination With Intrathecal Bupivacaine on Spinal Anesthesia:(https://pubmed.ncbi.nlm.nih.gov/32222361/) A Systematic Review and Meta-analysis. Clin Ther. 2020 Apr;42(4):676-690.e5. doi: 10.1016/j.clinthera.2020.02.009. Epub 2020 Mar 25. PMID: 32222361.
- Khosravi F, Sharifi M, Jarineshin H. Comparative Study of Fentanyl vs Dexmedetomidine as Adjuvants to Intrathecal Bupivacaine in Cesarean Section: A Randomized, Double-Blind Clinical Trial. J Pain Res. 2020 Oct 7;13:2475-2482. doi: 10.2147/JPR.S265161. PMID: 33116789; PMCID: PMC7548853.(https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7548853/#:~:text=In%20the%20present%20study%20intrathecal,compared%20to%2025%20%CE%BCg%20fentanyl.)
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
- Epidural (& other neuraxial) labour analgesia. Dr Dominique van Dyk. Part II Anaesthesia Refresher Course2015 University of Cape Town
- Image: Novice Anaesthesia. (2021). Infographics. Retrieved April 24, 2025, from https://www.gasnovice.com/infographics
- ERAS® Society. Guidelines for antenatal and pre-operative care in caesarean delivery (Part 1). 2022. erassociety.org
- ERAS® Society. Guidelines for intra-operative care in caesarean delivery (Part 2). 2022. erassociety.org
- ERAS® Society & ACOG. Guidelines for post-operative care in caesarean delivery (Part 3). Am J Obstet Gynecol. 2025. ajog.org
Summaries
Labour Analgesia
Regional complications
Post dural puncture headache
—
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “9a444f85-0414-4d5f-ab8a-ed8d042d293d”






