- Total Intravenous Anaesthesia (TIVA) & Target-Controlled Infusion (TCI)
- Routine Clinical Use of TIVA & TCI
- TCI Model Comparison
{}
Total Intravenous Anaesthesia (TIVA) & Target-Controlled Infusion (TCI)
Why Choose TIVA?
| Domain | Key value propositions |
|---|---|
| Patient | MH susceptibility, dystrophy / myopathy, severe reactive airways, high PONV risk, emergence delirium, previous volatile allergy, mask phobia |
| Surgical | Neurosurgery, spine with MEP/SSEP monitoring, middle-ear, strabismus, airway laser, shared airway, day-case ENT, remote MRI / CT suite |
| Environmental | Low-flow theatre unavailable, volatile scavenging limitations, greenhouse-gas reduction (propofol GWP ≈ 0) |
Drug–device Ecosystem
- Infusion pump–CE-marked TCI capable; anti-siphon, free-flow valved set.
- pEEG monitor (BIS, SedLine, Entropy)–reduces over-dosage and awareness.
- Intravenous bolus injector–for manual TIVA (e.g., Paeds).
- Drugs
- Propofol (1 % or 2 % lipid emulsion)–hypnotic backbone.
- Short-acting opioid–remifentanil (TCI Minto/Alfentanil), alfentanil or fentanyl bolus.
- Adjuncts–dexmedetomidine, ketamine, lidocaine, magnesium.
Pharmacokinetic Models in Clinical Use
| Agent | Adult models (BMI guidance) | Paediatric models | Notes |
|---|---|---|---|
| Propofol | Marsh (weight-based); Schnider (uses age, height, lean body mass; accurate up to BMI < 35 ♀ / 42 ♂) | Paedfusor (age 1–16 yr), Kataria (≥ 3 yr) | Context-sensitive half-time ≤ 40 min after 8 h infusion |
| Remifentanil | Minto (uses LBW; unreliable BMI > 40) | Rigby-Jones (Paedrem) | Effect-site TCI mandatory due to ke0 0.26 min-¹ |
| Dexmedetomidine | Hannivoort (adult) | Potts | No approved TCI pumps yet (manual titration) |
- Obesity–If BMI > 35–40, use Adjusted BW (IBW + 0.4×[TBW–IBW]) for Marsh; Schneider tolerates moderate obesity but titrate to pEEG and haemodynamics.
Practical Set-up Checklist (“STEEP”)
- System–audited TCI pump, dedicated IV line with anti-reflux valve
- Target–define induction Ce (e.g., propofol 3–4 µg mL⁻¹ + remifentanil 2–3 ng mL⁻¹) & maintenance range; pEEG 40–60
- Equipment–spare battery, extension sets, filters, infusion log
- Emergency–manual propofol syringe, volatile back-up, vasopressors, bag-valve set
- Plan–weight, height, age entered correctly; induction sequence briefed
Dosing Pearls
Propofol (adult)
| Phase | Typical Ce | Notes |
|---|---|---|
| Induction | 3–4 µg mL⁻¹ (Schnider) | Raise by 0.5 µg mL⁻¹ every 30 s if inadequate loss of eyelash reflex |
| LMA maintenance | 2–3 µg mL⁻¹ with remifentanil 1–2 ng mL⁻¹ | Keep pEEG 45–60 |
| Intubated major surgery | 3–5 µg mL⁻¹ + remifentanil 3–6 ng mL⁻¹ | Haemodynamic-guided |
Propofol Dose-sparing Factors
- Remifentanil, dexmedetomidine, ketamine 0.25 mg kg⁻¹, nitrous 50 % ↓ Ce by ~50 %.
Advantages & Limitations
| Advantages | Challenges |
|---|---|
| Stable ICP & cerebral autoregulation, less PONV, rapid cognitive recovery, immobility without trigger for MH, no atmospheric pollution | Requires reliable IV access & pumps, risk of awareness if line disconnects, hypotension (vasodilation), infusion-set deadspace error, drug cost (remifentanil) |
Safety & Troubleshooting
- Line disconnection–Use luer-locking anti-siphon set; audible occlusion alarms; pEEG ↑ trend & HR/BP spikes alert.
- Bolus artefact on start-up (Marsh)–use Gradual start function or set initial Ce rather than Cp.
- Bradycardia / hypotension–decrease propofol Ce by 0.3 µg mL⁻¹, give phenylephrine 100 µg; consider ketamine addition
- ECG interference by HFNO–ensure proper lead placement.
- Awareness prevention–pEEG monitoring, maintain remifentanil, record end-tidal propofol (in-line MIR devices, if available).
Key Pharmacology Equations
- Loading dose = Ce_target × Vc (L kg⁻¹ × weight)
- Maintenance rate (steady state) = Ce × Clearanc
- Context-sensitive half-time (CSHT)–time for plasma conc ↓ 50 % after stopping infusion; propofol CSHT rises modestly then plateaus, remifentanil remains < 5 min irrespective of duration.
Documentation Essentials
- Model & version, patient scalars (TBW, IBW, height), target Ce/Cp profiles, start/stop times, pEEG values, adjunct doses, pump log print-out (where possible).
Routine Clinical Use of TIVA & TCI
Practical Protocol for TCI Using Different Models
General Principles
- Remifentanil TCI targets:
- Minto ≈ Eleveld (reduce in elderly).
- Eleveld targets are lower than Marsh → Lower than Schnider for propofol during the first 10 min.
- Key point: Titrate to individual patient/procedure
Sedation
- Propofol TCI range: 0.5–2.0 µg/mL.
- Painful parts:
- Occasional pain:
- Alfentanil: 0.25-0.75 mg.
- Remifentanil: 0.2-0.5 µg/kg bolus.
- Continuous or frequent pain:
- Remifentanil infusion: TCI 0.5–2.0 ng/mL.
- Occasional pain:
- Key point: Always titrate for free airway–adequate spontaneous ventilation!
General Anaesthesia
- Start with:
- Propofol sedation and/or remifentanil for sensitivity testing.
- Propofol Induction Targets (TCI):
- Eleveld: 3.0 µg/mL.
- Schnider: 6.0* µg/mL.
- Marsh: 4-5* µg/mL.
- (For March and Schnider reduce to 3-4 µg/mL after unconsciousness, i.e., after a few minutes.).
- Remifentanil Targets:
- Laryngeal Mask Airway (LMA): 6 ng/mL.
- Endotracheal Tube (ETT): 10 ng/mL.
- Maintenance:
- Propofol 2.5-4 µg/mL.
- Remifentanil as needed (2-15 ng/mL).
Children TIVA Recipe
- Propofol 10mg/ml
- Bolus 3-5mg/kg over 3 min
- Start at 15-10mg/kg/hour for 30 min then 8-10mg/kg/hour
- This is equivalent to 3ug/ml effect site concentration.
- Remifentanil: Draw up solution to 1ug/kg/ml
- Bolus 1-5ug/kg
- Run at 6 ml/hour = 0.1ug/kg/min
- Precedex 4ug/ml
- Bolus: 0.51ug/kg bolus over 10 min
-
- 0.5ug/kg bolus has been shown to lead to faster recovery Use for short cases
-
- Target 0.5ug-1ug/kg/hour
Dose-setting Roadmap (“Titrate to effect”)
Quick-reference Target Table (adult ASA I–III)
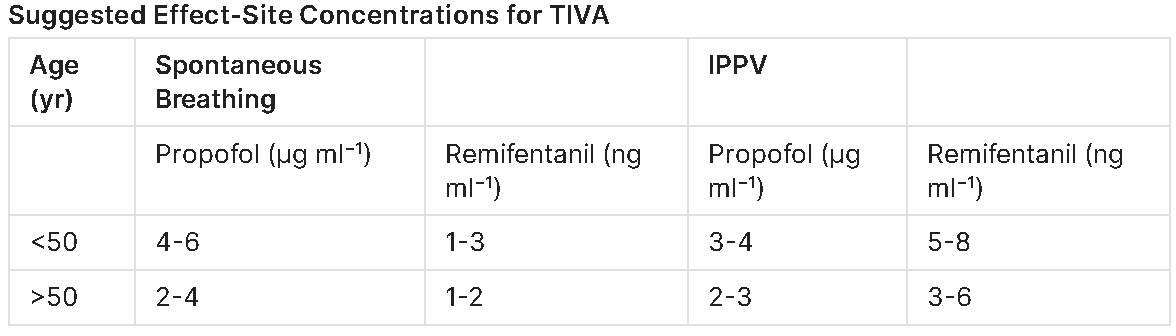
| Surgical stimulus | Propofol Ce (µg mL⁻¹)† | Remifentanil Ce (ng mL⁻¹)‡ | Sufentanil Ce (ng mL⁻¹) | Alfentanil Ce (ng mL⁻¹) |
|---|---|---|---|---|
| Skin incision | 3–4 (opioid co-admin) | 4–6 (LMA), 8–12 (ETT) | 0.3–0.4 | 60–70 |
| Maintenance – soft-tissue | 2–3 | 2–4 | 0.15–0.3 | 40–60 |
| Maintenance – abdominal/laparoscopy | 3–4 | 4–8 | 0.3–0.6 | 70–80 |
| Thoracic / major spine | 3–4 | 6–10 | 0.5–0.7 | 80–90 |
| Emergence / extubation | ↓ to 1.5 | ↓ to 1–2 (stop 5 min before) | 0.15 | 20–30 |
†Schnider/Marsh; reduce by 20 % for Eleveld in first 10 min.
‡Minto (adult LBW) or Eleveld; halve starting Ce in age > 70 yr.
Step-by-step Practical Protocol
Induction
- Assess & pre-oxygenate (head-up 20°, HFNO optional).
- Priming Ce
- Propofol target 1 µg mL⁻¹ → increase by 0.5 µg mL⁻¹ every 20–30 s while conversing with patient.
- Observe loss of response (LOR) → note Ce_LOR as guide (+0.5–1 for maintenance).
- Add opioid once Ce_propofol ≥ 2 µg mL⁻¹: set remifentanil Ce 4 ng mL⁻¹ (LMA) or 8 ng mL⁻¹ (ETT).
- NMBA when BIS < 55 or eyelash reflex lost.
Maintenance
- Hold propofol in a narrow band (± 0.3) around Ce producing BIS 45–60 and haemodynamic stability.
- Adjust opioid Ce to stimulus: skin closure often tolerates 2 ng mL⁻¹.
- Give anti-nociceptive adjuncts early (paracetamol, NSAID, dexamethasone, ketamine 0.25 mg kg⁻¹)
Emergence
- 15 min before end: decrease opioid Ce in two steps to 1–2 ng mL⁻¹; stop remifentanil 3–5 min pre-extubation or give morphine/hydromorphone to cover postoperative pain.
- Reduce propofol Ce to 1.5–2 µg mL⁻¹; keep BIS 60–70 until surgical dressing complete, then switch pump to stand-by.
- Ensure spontaneous ventilation, obeys commands → extubate.
Paediatric manual-TIVA Recipe (weight > 4 kg)
| Drug | Bolus | Infusion algorithm |
|---|---|---|
| Propofol 10 mg mL⁻¹ | 3–5 mg kg⁻¹ over 2–3 min | 15 mg kg⁻¹ h⁻¹ × 30 min → 10 mg kg⁻¹ h⁻¹ × 30 min → 8 mg kg⁻¹ h⁻¹ |
| Remifentanil (1 µg kg⁻¹ mL⁻¹) | 1 µg kg⁻¹ | 6 mL h⁻¹ (0.1 µg kg⁻¹ min⁻¹); titrate 4–8 mL h⁻¹ |
| Dexmedetomidine 4 µg mL⁻¹ | 0.5 µg kg⁻¹ over 10 min (optional) | 0.5–1 µg kg⁻¹ h⁻¹ for analgesia/smoothing |
Special Populations
| Group | Practical adjustment |
|---|---|
| Elderly > 70 yr | Start propofol Ce 0.8 µg mL⁻¹; increase by 0.25 µg mL⁻¹; maximum usually 2.5–3 |
| Morbid obesity (BMI > 40) | Marsh with adjusted BW; BIS mandatory; keep head-up to avert apnoea |
| Haemodynamically fragile | Ketamine-propofol admixture (“Ketofol” propofol 1 mg kg⁻¹ + ketamine 0.5 mg kg⁻¹) then 2 µg mL⁻¹ |
| Renal failure | Remifentanil safe (ester hydrolysis); avoid alfentanil accumulation; reduce dexmedetomidine 50 % |
Troubleshooting
| Issue | Likely cause | Fix |
|---|---|---|
| Hypotension, BIS < 40 | Propofol overdose | Step Ce down 0.3; fluid/vasopressor |
| Hypertension, BIS 50 | Inadequate analgesia | ↑ remifentanil Ce 2 ng mL⁻¹ or give bolus |
| Tachycardia + high BIS | Under-dosed both components | Check line patency; ↑ both Ce small increments |
| Slow awakening | Residual opioid ± high propofol | Stop opioid early; ensure Ce_propofol < 1.5 |
Key Safety Messages
- Titrate—not target: predicted Ce is a starting point, not a truth.
- pEEG (BIS/Entropy) + haemodynamics gives most reliable depth guide.
- Keep total lidocaine, propofol and opioid logs to detect infusion overrun.
- In-line anti-siphon valve and occlusion alarms prevent awareness from disconnection.
- Document model, scalars, Ce profile and any deviations.
TCI Model Comparison
| Model | Drug(s) | Population on which model was derived | Key covariates required by pump | Compartment structure | Main strengths | Principal limitations / caveats in routine use |
|---|---|---|---|---|---|---|
| Schneider (1998) | Propofol | 24 healthy adults (20–53 yr, BMI < 30 kg m-²)–arterial sampling | Age, height, total body-weight (pump calculates lean body mass) | 3-compartment, ke0 included | • Effect-site (Ce) targeting built-in • Lower initial bolus (Vc ≈ 4 L) → gentler induction |
• Small, narrow cohort → accuracy falls with obesity, paediatrics, elderly > 65 yr • Assumes normal cardiac output • Over-estimates dose if extreme lean or obese because of James LBW equation bias |
| Marsh (1991) | Propofol | 18 healthy adults (18–84 yr)–venous sampling | Total body-weight only | 3-compartment, no ke0 (pump estimates) | • Simple to program; widely validated • Good for prolonged infusions |
• Larger central V (≈ 16 L) ⇒ high induction bolus & overshoot • No age covariate → risk of overdose in frail/elderly • Inaccurate with children <16 yr |
| Minto (1997) | Remifentanil | 40 volunteers (20–85 yr; BMI < 35)–arterial sampling | Age, height, sex, lean body-weight (LBW) | 3-compartment with effect-site | • Excellent correlation of Ce with µ-opioid effect • Rapid titration; broad adult age range |
• LBW (James) unreliable when BMI > 40 kg m-² → under-dosing (“Ce gap”) • Not validated <12 yr or pregnancy |
| Gepts (1995) | Sufentanil (also alfentanil data set) | 113 adults from mixed surgical studies (normo- & overweight) | Total body-weight (allometric scaling) | 3-compartment | • Pooled real-surgery data → good for intra-operative dosing • Compatible with effect-site mode on modern pumps |
• Model files proprietary; availability varies by pump • Less robust in extremes of age/weight; no paediatric data |
| Eleveld (2018–2020) | Propofol and Remifentanil | Very large pooled database–5 227 propofol & 2 211 remifentanil subjects (premature neonates → 88 yr; 0.8–160 kg; ASA 1–IV; various ethnicities) | Age, sex, height, total body-weight (allometric scaling incorporated); no manual LBW entry | 3-compartment with allometric size & maturation functions; separate ke0 for awake vs anaesthetised | • First truly “all-age” unified model (preterm neonate → extreme obesity) • Better predictive performance at population outliers than Marsh**/Schnider/Minto • Same mathematical structure for propofol and remifentanil → synchronised dosing |
• Requires latest pump software (not yet on all devices) • Slightly slower equilibration → higher initial Ce needed for rapid induction • Limited peer-reviewed clinical data beyond 2022; still learning optimal target ranges |
Eleveld model—creation & Practical Advantages
| Aspect | Details |
|---|---|
| Data set & methodology | Developed by Prof. Djamel Eleveld & colleagues (2018–20) using NONMEM® to pool > 7 400 PK/PD profiles collected worldwide. It spans premature neonates (post-menstrual age 28 weeks) to octogenarians, total body-weight 0.8–160 kg and ASA classes I–IV. Weight and age effects were incorporated via allometric scaling (weight^¾ for clearance, weight^1 for volumes) and sigmoid maturation functions for infants. |
| Unified structure | The same 3-compartment model form and covariate equations were fitted separately to propofol and remifentanil, allowing parallel TCI with identical size/age corrections—unique among commercial models. |
| Effect-site (ke0) | Separate ke0 parameters for the awake state (induction) and anaesthetised state (maintenance) improve prediction of onset/offset dynamics. |
| Benefits compared with older models | • Broader applicability–valid from neonates to super-obese adults, reducing need to switch models. • Automatic scaling–no manual lean-body-weight calculation; avoids James LBW errors in obesity. • Improved bias/precision–validation shows median prediction error < 15 % across all subgroups, outperforming Marsh/Schnider in elderly and obese cohorts. • Consistent remifentanil–propofol interaction–same covariate philosophy simplifies teaching & usage. • Safety–lower risk of inadvertent underor over-dosing at patient extremes; complements pEEG-guided titration. |
The Eleveld model is a pharmacokinetic-pharmacodynamic (PK-PD) model developed to optimize propofol dosing across diverse patient populations, including children, adults, the elderly, and obese individuals. This model was constructed using data from a wide age range (3–90 years) and body weights (12–160 kg), allowing it to adjust dosing based on variables such as age, weight, sex, and the concurrent use of opioids. Eleveld has usefully been used in all age groups
In contrast, the Marsh and Schnider models are traditional PK models used in target-controlled infusion (TCI) systems for propofol administration:
- Marsh Model: Developed from studies involving healthy adults with a limited range of weights, this model considers total body weight for dosing calculations but does not account for age or lean body mass, which can lead to inaccuracies, especially in obese patients.
- Schnider Model: This model incorporates factors such as age, height, weight, and lean body mass, offering improved dosing accuracy over the Marsh model. However, it has limitations in patients with higher body mass indices (BMI), as the lean body mass calculation becomes less reliable in these cases.
Key Pharmacokinetic Parameters:
- Marsh Model: The central compartment volume (V1) is calculated as 0.228 L/kg, resulting in a V1 of approximately 15.96 L for a 70 kg individual.
- Schnider Model: The central compartment volume (V1) is fixed at 4.27 L, regardless of patient weight.
- Eleveld Model: This model does not rely on fixed compartment volumes; instead, it uses covariate-based equations to adjust parameters dynamically based on individual patient characteristics, such as age, weight, and sex.
Benefits of the Eleveld Model:
- Comprehensive Applicability: Validated for use across a wide range of patient populations, including children, adults, the elderly, and obese patients, eliminating the need for multiple models.
- Individualized Dosing: By incorporating variables such as age, weight, sex, and co-administration of opioids, the Eleveld model provides more personalized dosing regimens, potentially improving anesthetic efficacy and safety.
- Enhanced Accuracy: Prospective clinical validation studies have demonstrated that the Eleveld model achieves a predictive precision of less than 30% for arterial plasma concentrations and bispectral index (BIS) predictions, with a low population bias when used in TCI during clinical anesthesia practice.
Manual Infusions
Anaesthesia
| Drug | Loading Dose (µg/kg) | Maintenance Infusion (µg/kg/min) | Maintenance Infusion (µg/kg/h) |
|---|---|---|---|
| Alfentanil | 50-150 | 0.5-3 | 30-90 |
| Fentanyl | 5-15 | 0.03-0.1 | 1.8-6.0 |
| Sufentanil | 0.5-5 | 0.01-0.05 | 0.6-3.0 |
| Remifentanil | 0.5-1.0 | 0.1-0.4 | 6-24 |
| Ketamine | 1500-2500 | 25-75 | 1500-4500 |
| Propofol | 1000-2000 | 50-150 | 3-9 |
| Midazolam | 50-150 | 0.25-1.5 | 15-90 |
Sedation or Analgesia
| Drug | Loading Dose (µg/kg) | Maintenance Infusion (µg/kg/min) | Maintenance Infusion (µg/kg/h) |
|---|---|---|---|
| Alfentanil | 10-25 | 0.25-1 | 15-60 |
| Fentanyl | 1-3 | 0.01-0.03 | 0.6-1.8 |
| Sufentanil | 0.1-0.5 | 0.005-0.01 | 0.3-0.6 |
| Remifentanil | † | 0.025-0.1 | 1.5-6 |
| Ketamine | 500-1000 | 10-20 | 600-1200 |
| Propofol | 250-1000 | 10-50 | 600-3000 |
| Midazolam | 25-100 | 0.25-1 | 15-60 |
Notes on Infusion Protocols
General Guidelines
- After the loading dose, an initially high infusion rate to account for redistribution should be used. This rate should then be titrated to the lowest infusion rate that will maintain adequate anesthesia or sedation.
- When using opiates as part of a nitrous-narcotic technique or for cardiac anaesthesia, the dosing scheme listed under anaesthesia is used.
- When the opiate is combined as part of balanced anaesthesia, dosing listed for analgesia is needed.
Remifentanil Specific Note
- For analgesia or during sedation, an initial loading dose of remifentanil should not be given because its very rapid onset may result in apnoea or muscle rigidity.
Practical Aspects of TCI / TIVA
Avoiding Accidental Awareness
| Root cause | Practical counter-measure |
|---|---|
| Drug not delivered • Disconnection / infiltration • Empty syringe, silent KVO rate |
• Dedicated anti-siphon/anti-reflux TIVA set, Luer-lock throughout • Visible IV site at all times (even in MRI / prone) • Audible occlusion & empty-syringe alarms activated • NEVER use pumps with automatic KVO mode for propofol/remifentanil |
| Pharmacology misunderstood (wrong model, weight scalar, inappropriate target) |
• Standardise to one pump & model set per department (e.g. Schnider ≤ BMI 35/42; Eleveld for extremes) • pEEG guidance when NMB used • Record Ce at loss-of-response as individual calibration |
Drug Concentrations, Pumps & Syringes
| Item | Departmental standard | Safety rationale |
|---|---|---|
| Propofol | Single concentration (1 % or 2 %) | Eliminates dosing error |
| Remifentanil | Dilute to 20 µg mL⁻¹ (or 50 µg mL⁻¹ paeds) | Consistent TCI programming & manual maths |
| TCI pump | One model, locally validated PK set; annual service | Training & firmware uniformity |
| Syringe | One ISO-compliant brand, Luer-lock | Prevents mis-recognition and disconnection |
| Alarms | Occlusion ± drop-pressure, empty, battery, mains | Rapid fault detection |
| Visual indicators | Infusion-running light or screen | Confirms delivery in low-noise MRI/ICU |
| Mixing drugs | Do not mix propofol + remifentanil | Phase-separation, unpredictable dosing; breaches licence |
- Tip: programme the syringe type after it is seated; prevents mismatch.
Infusion Sets & Cannulae
- Dedicated 2or 3-way TIVA set:
- Anti-siphon valve on drug lumen
- Non-return valve on fluid lumen
- < 0.3 mL dead-space distal to junction
- Join lines as close to cannula as practical.
- Secure all connections; keep line visible; document site checks hourly.
Preparation Checklist (Mnemonic “PUMP-SAFE”)
- Power–mains plugged, battery > 80 %.
- Update–correct model & firmware.
- Medicine–syringe filled, labelled after draw-up, right dilution.
- Pump programming–drug, concentration, syringe type match.
- Set–dedicated anti-siphon line, no extra hubs.
- Alarm limits–occlusion 300–500 mmHg; battery; disconnection.
- Fail-plan–manual propofol syringe ready; volatile back-up.
- Enter patient data–age, weight (adjusted BW if BMI > 35), height.
Conduct of TIVA
Start-up
- Run pEEG before induction; note baseline.
- Observe initial infusion rate (mg kg⁻¹ h⁻¹)–corroborates correct entry.
- Give NMB only after BIS < 60 and Ce stable for 60 s.
During case
- Review pump screen _q_15 min: predicted Ce, syringe volume, rate.
- Record pEEG and haemodynamics; titrate Ce in 0.2-0.5 increments.
- Alarm action rule: STOP → check cannula site → flush → resume
Pump Failure
- Note last rate (ml h⁻¹).
- Switch to manual mode at same rate (or 100 µg kg⁻¹ min⁻¹ remi = 6 ml h⁻¹ of 20 µg mL⁻¹).
- Re-boot or replace pump; recommence TCI with new target titrated from observed BIS.
End of case
- Stop remifentanil 3–5 min before extubation (or give longer-acting opioid).
- Reduce propofol Ce to BIS 60–70.
- Flush drug line and cannula with ≥ 5 mL saline (≥ 2× dead-space).
Monitoring
| Monitor | Minimum during TIVA | Comment |
|---|---|---|
| pEEG (BIS/Entropy/SedLine) | Mandatory if paralysis used | Start pre-induction, stop after TOF > 0.9 |
| Neuromuscular | TOF every 15 min | Ensures paralysis not masking AAGA |
| Standard ASA | ECG, NIBP/IBP, SpO₂, capnography, temp | Same for remote/MRI units |
Special Situations
| Scenario | Practical adjustments |
|---|---|
| Rapid sequence | Propofol TCI: set Ce 6 µg mL⁻¹ (Schnider) or 3 µg mL⁻¹ (Eleveld + remi) and hit start/fast. Reduce to 3–4 µg mL⁻¹ after tube placed. |
| Switch volatile → TIVA | Start propofol TCI while MAC ≈ 0.6; increase Ce as ETAA falls; use pEEG to avoid awareness dip. |
| MRI suite | Use MR-conditional pump outside 0.5 mT line; 400 cm low-compliance line; visual line-of-sight through window; add wireless SpO₂ & NIBP. |
| Extreme obesity | Adjusted body-weight for Marsh; prefer Eleveld; head-elevated ramp; pEEG essential; HFNO for pre-oxygenation. |
Key Take-home Rules
- Dedicated line, dedicated pump, dedicated brain monitor.
- Insert syringe → label → programme (never vice-versa).
- Keep IV site visible; every alarm = look first, touch later.
- Flush out potent agents before patient leaves theatre.
- pEEG when NMB used; aim BIS 40–60 (adult), Entropy 40–60.
- Have a rescue plan: manual infusion or volatile at the ready.
Obesity & Model Choice
| BMI | Propofol model advice | Remifentanil model advice |
|---|---|---|
| ≤ 35 (♀) / 42 (♂) | Schnider acceptable (enter TBW) | Minto with LBW |
| > 35–55 | Marsh with adjusted BW or Eleveld (TBW) | Eleveld (TBW); Minto under-predicts |
| > 55 or extremes (paeds/geri) | Eleveld (validated 0.8–160 kg) | Eleveld |
Links
- Sedation
- Day case surgery
- Awareness
- Paediatric Total Intravenous Anaesthesia (TIVA)
- Practical Protocols and recipes
References:
- Anderson, B. J. and Houghton, J. (2019). Total intravenous anesthesia and target-controlled infusion. A Practice of Anesthesia for Infants and Children, 177-198.e3. https://doi.org/10.1016/b978-0-323-42974-0.00008-2
- Nimmo, A. F., Absalom, A., Bagshaw, O., Biswas, A., Cook, T., Costello, A., … & Wiles, M. D. (2018). Guidelines for the safe practice of total intravenous anaesthesia (tiva). Anaesthesia, 74(2), 211-224. https://doi.org/10.1111/anae.14428
- Al-Rifai, Z. and Mulvey, D. A. (2016). Principles of total intravenous anaesthesia: practical aspects of using total intravenous anaesthesia. BJA Education, 16(8), 276-280. https://doi.org/10.1093/bjaed/mkv074
- McGrenaghan, E. and Wilson, M. (2019). Total intravenous anaesthesia. Anaesthesia &Amp; Intensive Care Medicine, 20(2), 130-135. https://doi.org/10.1016/j.mpaic.2018.12.010
- AAGBI. Guidelines for Total Intravenous Anaesthesia (Second Edition). London, 2022
- DAS–AAGBI–RCoA. Preventing Accidental Awareness under General Anaesthesia (2023 update).
- Eleveld DJ, et al. An allometric all-age pharmacokinetic model for propofol & remifentanil. Anesthesiology. 2020;133:633-648.
- Brice A, et al. Best practice for infusion lines in TIVA. Anaesthesia. 2024;79:947-955.
- Hill A, et al. Propofol TCI in morbid obesity: a systematic review. BJA. 2024;133:1123-1132.
- AAGBI. Guidelines for TIVA and TCI in adults and children. 2nd ed. London, 2022.
- Absalom AR, Struys MMRF. Total Intravenous Anaesthesia and Target-Controlled Infusions. Springer; 2023.
- Vellinga R, et al. pEEG-guided versus fixed-target TIVA: randomised trial. Br J Anaesth. 2023;131:879-887.
- Berkenstadt H, et al. Remifentanil–propofol interaction revisited—a response surface analysis. Anesthesiology. 2025;142:225-234.
Summaries:
Copyright**
© 2025 Francois Uys. All Rights Reserved.
id: “5ef4ebce-4571-4e03-9c48-953778e235f6”



