- Introduction
- Core Principles for Anaesthesia
- Intravenous Induction Agents
- Volatiles
- N20
- Muscle Relaxants
- Anticholinergics
- Anticholinesterase Inhibitors
- Suggamadex
- Benzodiazepines
- Benefits of Alternatives
- Mechanism of Action
- General Safety
- Clinical Applications and Dosing by Route
- 1. Intravenous (IV) Use in Obstetric Anesthesia
- 2. Intrathecal Dexmedetomidine
- 3. Epidural Dexmedetomidine
- A. Epidural Conversion for Cesarean Section
- B. Epidural Top-Ups (Second Stage Labor)
- C. Continuous Labor Epidural Infusions
- 4. Peripheral Nerve Blocks
- 5. Nebulized Dexmedetomidine for Post-Dural Puncture Headache (PDPH)
- Dexmedetomidine as an Adjuvant in Regional Anesthesia
{}
Introduction
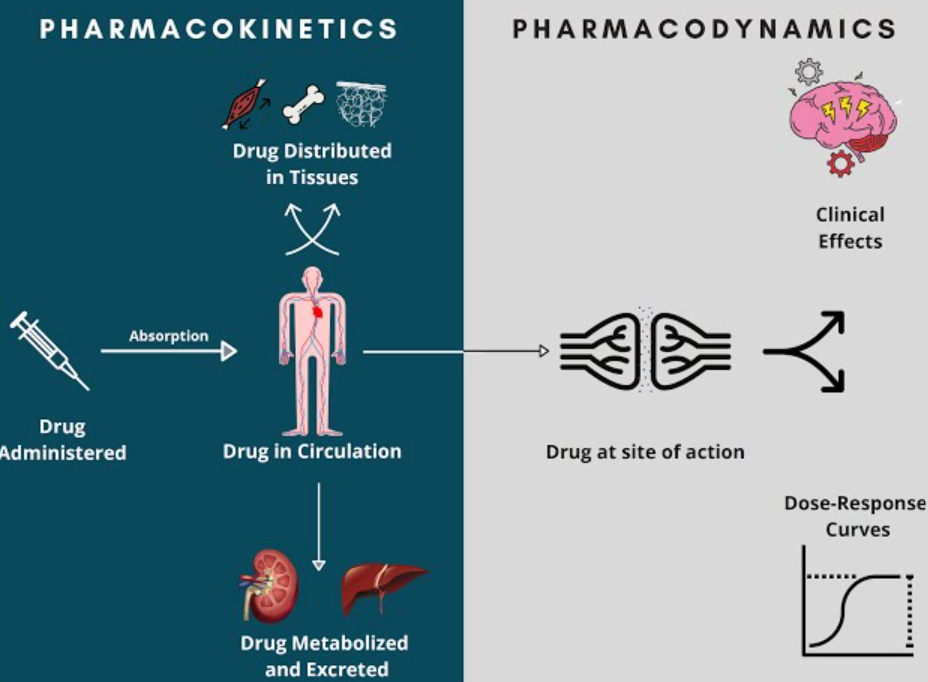
Core Principles for Anaesthesia
Fundamental Formulae
| Variable | Equation | Key points & units |
|---|---|---|
| Volume of distribution (Vd) | Vd = Dose ÷ Cp (L) | Apparent rather than anatomical; ↑ with lipophilic or tissue-bound drugs |
| Clearance (Cl) | Cl = Rateelim ÷ Cp (mL min⁻¹ or L h⁻¹) | Sum of all eliminating organs |
| Steady-state clearance (Clss) | Clss = Maintenance dose ÷ Css | At steady state: Ratein = Rateout |
| Organ clearance | Clorgan = Q × EH | Q = blood flow; EH = (Cin − Cout) ÷ Cin |
| Hepatic extraction ratio (EH) | 0–1; high (>0.7) vs low (<0.3) extraction drugs | Clhepatic ≈ Q for high-E drugs; ≈ fu × Clint for low-E |
| Bio-availability (F) | F = AUCoral ÷ AUCIV | Corrected for dose |
| Elimination half-life (t½) | t½ = 0.693 × Vd ÷ Cl | Five half-lives → 97 % eliminated |
| Loading dose | Loading = Css,target × Vd | Adjust for F if non-IV |
AUC = area under the plasma-concentration-time curve; Cp = plasma concentration; Css = steady-state concentration; fu = unbound fraction; Clint = intrinsic clearance.
Pharmacodynamics
| Concept | Anaesthetic example | Clinical relevance |
|---|---|---|
| Agonist | Propofol at GABAA | ↓ CNS excitability, hypnosis |
| Competitive antagonist | Rocuronium at nicotinic ACh receptor | Paralysis overcome by ↑ ACh (sugammadex reverses) |
| Non-competitive antagonist | Ketamine at NMDA receptor | Dissociative anaesthesia; analgesia |
| Efficacy (Emax) | Ceiling respiratory depression of partial μ-agonists | Determines maximal attainable effect |
| Potency (EC50) | Fentanyl ≪ morphine | Choice of dose & infusion rate |
| Therapeutic index (TI) | Volatile agents (wide) vs thiopental (narrow) | Guides safe practice |
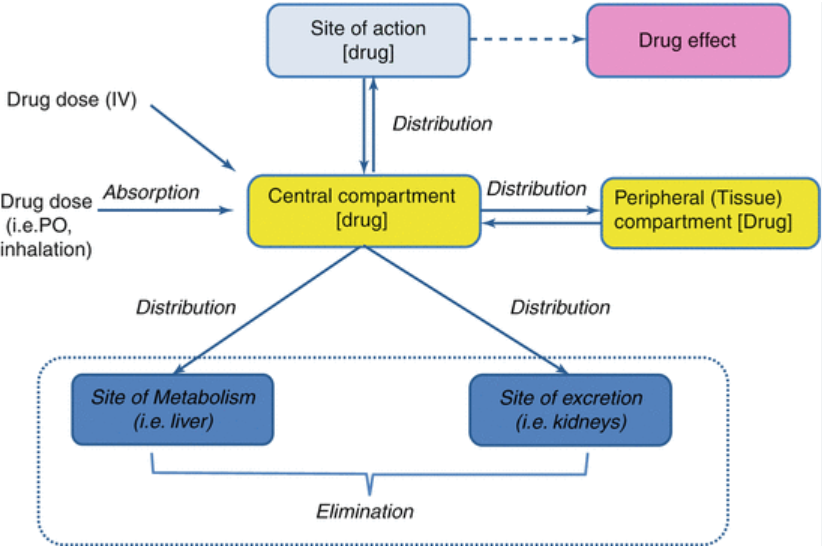
Pharmacokinetics
Absorption
- IV—F ≈ 1; rapid onset (propofol, lidocaine).
- Inhaled—uptake governed by Fick’s law; blood–gas solubility drives speed (desflurane fastest).
- Enteral—subject to first-pass metabolism; pro-drugs (codeine → morphine via CYP2D6).
- Topical/rectal—variable; superior haemorrhoidal vein drains to portal system.
Distribution
- Large Vd for lipophilic drugs (fentanyl > 4 L kg⁻¹).
- Reduced α1-acid glycoprotein in neonates → ↑ free local anaesthetic.
- Two-compartment kinetics explain rapid awakening after a single bolus of propofol.
Metabolism
| Phase | Reaction | Examples | Notes |
|---|---|---|---|
| I | Oxidation, reduction, hydrolysis (CYP450) | Midazolam (CYP3A4), ester hydrolysis of remifentanil | Unmask/enhance polarity |
| II | Conjugation (glucuronidation, sulphation) | Morphine-3-glucuronide, lorazepam | Usually inactive, renal excretion |
- Inducers—PC BRAS: Phenytoin, Carbamazepine, Barbiturates, Rifampicin, Alcohol (chronic), St John’s wort/Smoking.
- Inhibitors—SICKFACES.COM: Sodium valproate, Isoniazid, Cimetidine, Ketoconazole, Fluconazole, Alcohol (binge), Chloramphenicol, Erythromycin, Sulphonamides, Ciprofloxacin, Omeprazole, Metronidazole.
Excretion
- Renal—filtration (free drug < 30 kDa), secretion (OCT/OAT transporters), re-absorption (pH-dependent).
- Biliary/enteric—phase II conjugates (morphine-6-glu) undergo entero-hepatic recycling.
- Pulmonary—principal route for volatile agents; governed by solubility and minute ventilation.
Context-Sensitive Half-Time (CSHT)
- Time for plasma concentration to fall 50 % after stopping an infusion.
- Remifentanil CSHT < 5 min even after 4 h; fentanyl CSHT > 200 min after 4 h.
- Guides drug selection for target-controlled infusion (TCI).
Drug Interactions
| Interaction | Example | Practical implication |
|---|---|---|
| Additive | Sevoflurane + N₂O | Reduced volatile requirement |
| Synergistic | Propofol + midazolam | Marked hypotension—dose-reduce |
| Potentiation | Clavulanate potentiates amoxicillin | Not dose-sparing for amoxicillin toxicity |
| Antagonism | Naloxone vs μ-opioids | Reversal of respiratory depression |
Acid–Base & Ionisation (Henderson–Hasselbalch)
- Only the non-ionised fraction diffuses rapidly across the BBB and placenta.
- pKa of local anaesthetics ~7.8–8.9: tissue acidosis → delayed block onset.
Take-Home
- High-extraction drugs (e.g., propofol) are flow-limited; changes in hepatic blood flow have greater impact than enzyme induction.
- Low-extraction drugs (e.g., phenytoin) are capacity-limited; clearance altered by protein binding and enzyme activity.
- Five half-lives to steady state; but with TCI the context-sensitive profile is more clinically useful.
- CYP induction/inhibition is a frequent cause of peri-operative drug failure or toxicity (e.g., erythromycin prolonging midazolam sedation).
- Therapeutic index informs safe practice: volatile anaesthetics (TI ≈ 2–4) vs neuromuscular blockers (TI ≈ 2).
Intravenous Induction Agents
| Feature | Etomidate | Propofol | Ketamine |
|---|---|---|---|
| Typical induction dose | 0.2–0.3 mg kg⁻¹ | 1.5–2.5 mg kg⁻¹ (reduce in frail/older pts) | 1–2 mg kg⁻¹ IV (4–6 mg kg⁻¹ IM) |
| Onset / duration | 30 s / 3–5 min | 20 s / 5–10 min | 45 s / 10–15 min |
| Haemodynamics | Stable: minimal ↓ SVR or contractility | ↓ SVR, preload & contractility → hypotension, brady-flex blunted | Sympathomimetic ↑ HR, BP, CO (may fall in catecholamine-depleted states) |
| Respiration | Mild depression, apnoea uncommon | Dose-dependent respiratory depression & apnoea | Preserves pharyngeal tone & resp drive; bronchodilation |
| Cerebral effects | ↓ CMRO₂, CBF & ICP | ↓ CMRO₂, CBF & ICP | ↑ CBF & ICP; maintains CPP via ↑ MAP |
| Analgesia | None | None | Profound (NMDA blockade) |
| Adrenocortical suppression | Blocks 11-β- & 17-α-hydroxylase for 6-24 h after single dose | None | None |
| Other issues | Myoclonus (↓ with midazolam/opioid), injection pain, N/V | Pain on injection (lidocaine/opioid pre-treat), propofol infusion syndrome (see below) | Emergence delirium → ↓ with midazolam/α-2-agonist; hypersalivation (glycopyrrolate) |
Etomidate
Pharmacology
- Carboxylated imidazole, 75 % protein-bound, Vd ≈ 2.5 L kg⁻¹.
- Rapid hepatic/esterase metabolism → inactive carboxylic acid; t½β ≈ 3 h
Clinical Use
- Induction in haemodynamically unstable trauma, severe AS, cardiogenic shock, raised ICP.
- Avoid repeated doses or continuous infusions in sepsis/critical illness owing to adrenocortical suppression; single bolus has not shown mortality harm in large RCTs but blunts cortisol response for up to 24 h.
Adverse Effects & Mitigation
| Effect | Mechanism | Prevention / management |
|---|---|---|
| Myoclonus (30–60 %) | Disinhibition of subcortical centres | 1 mg midazolam or 0.5 µg kg⁻¹ remifentanil pre-induction |
| Adrenal suppression | 11-β-/17-α-hydroxylase inhibition | Single bolus only; consider stress-dose steroids if septic shock |
| Injection pain | TRPA1 activation | Lidocaine 20 mg + large-bore cannula |
| PONV | Stimulation of CTZ | Prophylactic anti-emetic |
Propofol
Pharmacology
- 1 % lipid emulsion; 98 % protein bound, Vd ≈ 3–4 L kg⁻¹.
- Rapid redistribution (awakening 5–10 min); hepatic glucuronidation + extra-hepatic clearance (lungs, kidneys)
- Context-sensitive half-time ≤ 40 min for infusions < 8 h.
Physiological Effects
- CNS–global depression, anticonvulsant, ↓ ICP; profound amnesia.
- CVS–vasodilatory hypotension (greater if hypovolaemic, elderly, β-blocked).
- Respiratory–dose-dependent depression with loss of protective reflexes.
- Anti-emetic–useful for TIVA or rescue PONV bolus 10–20 mg.
Propofol Infusion Syndrome (Prise)
- Risk factors: dose > 4 mg kg⁻¹ h⁻¹ for > 48 h, paediatrics, catecholamines, steroids, critical illness, mitochondrial disease.
- Early clues: metabolic/lactic acidosis, rising CK / TGs, Brugada-like ECG.
- Management: stop propofol, support circulation, renal replacement, ECMO if refractory.
Ketamine
Pharmacology
- Racemic mixture; S-ketamine more potent. 20–50 % protein bound, Vd ≈ 3 L kg⁻¹.
- Hepatic N-demethylation → norketamine (active, 30 % potency); t½β ≈ 2–3 h.
Mechanisms
- Non-competitive NMDA antagonism → dissociative anaesthesia, analgesia, anti-hyperalgesia.
- Weak μ- and κ-opioid agonism, monoamine re-uptake inhibition, muscarinic blockade, HCN1 channel inhibition.
Clinical Advantages
- Haemodynamic support in shocked patients.
- Bronchodilation in status asthmaticus.
- Opioid-sparing in multimodal analgesia and chronic pain.
- Rapid-acting antidepressant (sub-anaesthetic doses).
Adverse Effects & Mitigation
| Effect | Management |
|---|---|
| Emergence delirium, hallucinations (10–20 %) | Midazolam 0.02 mg kg⁻¹; quiet environment; α-2-agonist |
| Hypersalivation | Glycopyrrolate 4–6 µg kg⁻¹ IV |
| ↑ ICP / IOP (theoretical) | Acceptable if MAP ensured; caution in space-occupying lesion |
| Cystitis with chronic abuse | Counsel long-term users |
Volatiles
Volatile Gases
Overview
- Examples: Desflurane, sevoflurane, isoflurane.
- Primary Indication: Facilitates induction and maintenance of general anesthesia.
- Route of Administration: Inhalation.
- Metabolism & Excretion: Minimal metabolism, excretion by exhalation.
Mechanism of Action
- Potentially interacts with GABA activated chloride channels to induce hyperpolarization and CNS depression.
- Potentially inhibits excitatory presynaptic channel activity mediated by neuronal nicotinic, serotonergic, and glutaminergic receptors.
- Potentially interacts with two-pore domain potassium channels to alter resting membrane potential of neurons.
Effects
- CNS
- Decreased cerebral metabolic rate.
- Decreased cerebral oxygen consumption.
- Results in loss of consciousness and amnesia.
- Cardiovascular
- Decreased systemic vascular resistance.
- Results in hypotension, tachycardia, and prolonged QT interval (especially sevoflurane).
- Respiratory
- Decreased hypoxic and hypercapnic respiratory drive.
- Decreased tidal volume and alveolar ventilation.
- Results in hypercapnia and increased respiratory rate.
- Musculoskeletal
- Neuromuscular blockade and potentiation of NDNMBs.
- Results in muscle relaxation and malignant hyperthermia.
Minimum Alveolar Concentration (MAC) and Blood-Gas Partition Coefficient (BGPC)
| Gas | MAC | BGPC |
|---|---|---|
| Halothane | 0.75 | 2.5 |
| Isoflurane | 1.15 | 1.4 |
| Sevoflurane | 2.0 | 0.64 |
| Desflurane | 6.0 | 0.42 |
Uptake Effect
- Physical properties of gas:
Molecular weight
Solubility (less soluble = faster onset) - Alveolar gas concentration:
Inspired fresh gas flow concentration
Ventilation
2nd gas effect
Uptake and degradation by anaesthetic delivery system - Cardiac output
MAC
- Minimum alveolar concentration required to prevent a gross muscular movement in response to a standard painful stimuli in 50% of patients after anaesthetic equilibrium is reached under standardized conditions.
- Painful stimuli= incision of set width and depth
- Standardised conditions = Sea level, normothermic, normotensive, normal oxygenation.
Desflurane
- Pro:
Fast offset =
Rapid emergenceparticularly in obese patients and prolonged procedures (although minimal in comparison to sevo and probably not clinically relevant)
Faster turnover (evidence is scanty) - Con:
Pungent odour = Respiratory irritant
Lower potency
Expensive
More potent vasodilator than sevo (less haemodynamically stable)
Environmental impact (1hr use is equivalent to 350miles drive)- NB: 1hr of sevo = 18mile drive.
N20
Overview
- Primary Use: Anesthesia Adjuvant.
- Secondary Use: Analgesia, Dental Sedation, Anxiolytic.
- Route of Administration: Inhalation.
- Metabolism: None.
- Excretion: Primarily lung (exhalation).
- Minimum Alveolar Concentration: >100% @1atm (Incomplete Anesthetic).
Mechanism of Action
- NMDA Receptor Antagonist
- Inhibition of NMDA receptor, leading to closure of NMDA receptor channel, inhibition of ionic currents, and decreased central nervous system excitability.
- Results in anesthesia.
- Modulation of Nociception
- Stimulation of neurons in PAG of midbrain causes release of EOPs and/or DYNs.
- Activation of opioid receptors in GABA-ergic nuclei of pons causes inhibition of inhibitory GABA-ergic pathway.
- Activation of descending noradrenergic system in spinal cord posterior grey column.
- Inhibition of primary afferent and second-order neuron nociception.
- Results in analgesia.
- GABA_A Receptor Activation
- Activation of GABA_A receptor benzodiazepine binding site causes increased chloride influx.
- Possible activation of CaM-NOS-cGMP-PKG pathway (exact mechanism unknown).
- Results in anxiolysis.
Side Effects
- Vitamin B12 Inactivation: N2O irreversibly oxidizes Vitamin B12 (cobalamin), leading to irreversible inhibition of methionine synthase and resulting in megaloblastic anemia.
- Pyramidal Cell Vacuole Reaction: Swelling of endoplasmic reticulum and mitochondria, causing reversible pyramidal cell neurotoxic vacuole reaction in posterior cingulate/retrosplenial cortex.
- Myeloneuropathy.
- Encephalopathy.
- Subacute Combined Degeneration of spinal cord.
- Peripheral Neuropathy.
- Hyperhomocysteinemia.
- Neurotoxicity.
Nitrous Oxide (N₂O) and Cardiovascular Risk
Theoretical Risk
- Homocysteine Levels:
- N₂O increases levels of homocysteine.
- Elevated homocysteine is associated with an increased risk for coronary artery disease (CAD) and cerebrovascular disease.
Clinical Evidence
- ENIGMA-2 Trial:
- Study involved 7000 high-risk patients.
- Assessed the intraoperative use of N₂O.
- Findings: N₂O use was not associated with an increased risk of death or cardiovascular complications within 30 days of surgery.
Considerations
- Long-Term Risk:
- The ENIGMA-2 trial did not address the risk of myocardial infarction (MI) occurring more than 30 days postoperatively.
Muscle Relaxants
Non-Depolarizing Neuromuscular Blockers (NDMBs)
Mechanism of Action
- Competitive antagonism at post-synaptic nicotinic receptors on muscles.
- Decreases binding sites for ACh, resulting in muscle cell depolarization and skeletal muscle paralysis.
- Competitive antagonism at pre-synaptic nicotinic receptors on neurons.
- Decreases positive feedback for continued ACh release in response to high-frequency stimulation, leading to tetanic fade and train-of-four fade.
Effects
- Vagolytic effect (especially pancuronium).
- Blockage of vagal muscarinic receptors in sinoatrial nodes.
- Decreases parasympathetic effects on the heart, resulting in tachycardia.
- Anaphylactic/anaphylactoid reactions.
- IgE antibodies attach to ammonium ion components of NDNMBs.
- Non-immunologic mast cell degranulation (especially atracurium).
- Results in bronchospasm and hypotension
Quick Facts
- Primary Indication: Skeletal muscle paralysis to facilitate tracheal intubation, used during indicated surgeries or mechanical ventilation.
- Route of Administration: IV.
- Metabolism & Excretion: Redistribution, hepatic clearance/renal excretion (extent varies greatly by drug). Not degraded by acetylcholinesterase or pseudocholinesterase.
Succinylcholine (Depolarizing Neuromuscular Blocker)
Mechanism of Action
- Succinylcholine mimics ACh in structure.
- Agonist at nicotinic ACh receptors in muscles.
- Generates action potential.
- Not affected by synaptic acetylcholinesterase (unlike ACh).
- Continuous end-plate depolarization.
- Inactivation of sodium channels.
- Prevention of repolarization and additional action potentials.
- Results in skeletal muscle paralysis.
Effects
- Agonist at nicotinic receptors in parasympathetic ganglia, sympathetic ganglia, and muscarinic receptors in SA node of the heart.
- Low dose: Parasympathetic effect, decreases heart rate and contractility.
- High dose: Sympathetic effects, increases heart rate, contractility, and catecholamine release.
- Serum K+ (especially in patients with muscle trauma/denervation/immobilization), leading to hyperkalemia and potential cardiac arrest.
Side Effects
- Fasciculation.
- Myalgia.
- Hyperkalemia.
- Malignant hyperthermia (see separate slide).
Quick Facts
- Primary Indication: Skeletal muscle paralysis to allow tracheal intubation with the advantage of faster onset (30s-60s) and shorter duration (<10min) than non-depolarizing neuromuscular blocking agents.
- Route of Administration: IV.
- Metabolism & Excretion: Redistribution and metabolism by pseudocholinesterase in blood plasma and liver.
- No reversal of succinylcholine available.
- Contraindicated in patients with traumatized, denervated, or immobilized muscles due to risk of cardiac arrest from hyperkalemia.
Scoline Apnea
Pathophysiology
- Acquired:
- Drugs (anticholinesterases): Break down or inhibit pseudocholinesterase enzyme, decreasing pseudocholinesterase activity.
- Malignancy: Abnormal gene expression decreases protein synthesis.
- Liver disease: Decreases liver’s ability to synthesize proteins, decreasing pseudocholinesterase synthesis by the liver.
- Malnutrition: Decreases molecular precursors for protein production.
- Renal disease: Mechanism unclear.
- Fluid overload: Hemodilution of circulating proteins.
- Hereditary:
- BChE (Butyrylcholinesterase) gene mutation: Decreases production or production of non-functional pseudocholinesterase by the liver.
Effect
- Impaired ability to hydrolyze ester linkages of substrates like neuromuscular blocking agents (e.g., succinylcholine, mivacurium, diamorphine, acetylsalicylic acid, methylprednisolone, cocaine, heroin).
- Increases patient’s susceptibility to side effects from drugs with ester linkages.
Anesthetic Considerations
- Prolonged binding of neuromuscular blocking agent to nicotinic cholinergic receptors in neuromuscular junctions.
- Prolonged depolarization of skeletal muscle, preventing repolarization and additional action potentials, resulting in extended muscle paralysis and/or anesthesia.
Note
- Pseudocholinesterase has many synonymous names including butyrylcholinesterase, BChE, BuChE, plasma esterase, plasma cholinesterase, and serum cholinesterase.
Drugs Administered
-
Mivacurium:
- Competitively binds to acetylcholine nicotinic receptor.
- Active site of post-synaptic acetylcholine receptor is blocked.
- Acetylcholine cannot act on receptor, skeletal muscle cannot depolarize, resulting in skeletal muscle paralysis.
-
Succinylcholine:
- Irreversibly binds to acetylcholine nicotinic receptor.
- Continuous depolarization of skeletal muscle.
- Skeletal muscle unable to repolarize, acetylcholine released cannot trigger an action potential, resulting in skeletal muscle paralysis.
Complications
- Since respiratory muscles are affected, apnea requires sedation and respiratory assistance for up to several hours.
Anticholinergics
Mechanism of Action
- Competitive antagonism at muscarinic receptors in various organ systems.
- Decreases binding sites for ACh at muscarinic receptors
Effects
-
Cardiovascular:
- Blockage of muscarinic receptors in SA node (especially atropine), resulting in tachycardia.
-
Respiratory:
- Smooth muscle relaxation in the bronchial airways, resulting in bronchodilation.
- Decreases respiratory tract mucosal secretions (especially glycopyrrolate, scopolamine), resulting in respiratory tract clearing.
-
Ophthalmic:
- Pupillary dilation, resulting in mydriasis.
-
Salivary:
- Decreases secretion of salivary glands, resulting in dry mouth.
-
Gastrointestinal:
- Decreases intestinal motility and peristalsis, resulting in constipation.
-
Genitourinary:
- Decreases ureter and bladder tone, resulting in urinary retention.
Quick Facts
- Primary Indication in the OR: Combined with acetylcholinesterase inhibitors to prevent over-activation of the parasympathetic nervous system.
- Route of Administration: IV.
Anticholinesterase Inhibitors
Mechanism of Action
- Reversible enzymatic inhibition of acetylcholinesterase.
- Decreases breakdown of ACh in neuromuscular junctions and rest of body.
Effects
- Neuromuscular Junction:
- Increases ACh available to compete with NDNMBs to bind to post-synaptic nicotinic receptors on muscles, re-establishing normal neuromuscular junction function.
- Increases ACh available to compete with NDNMBs to bind to pre-synaptic nicotinic receptors on neurons, increasing positive feedback for continued ACh release.
- Systemic:
- Stimulation of muscarinic receptors in various organ systems.
- Muscarinic (parasympathetic) effects:
- Hypotension.
- Bradycardia.
- Bronchoconstriction.
- Bronchial secretions.
- Salivation.
- Peristalsis.
- Nausea/vomiting.
Side Effects
- Excess dose leads to depolarizing block of the nicotinic receptors.
- Flaccid skeletal muscle paralysis.
- Respiratory paralysis and failure.
Quick Facts
- Primary Indication in the OR: Used as a reversal agent against non-depolarizing neuromuscular blockers post-surgery.
- Route of Administration: IV.
- Metabolism & Excretion: Hepatic clearance and renal excretion.
Suggamadex
Mechanism of Action and Adverse Side Effects
Mechanism of Action
- Binds rocuronium and vecuronium (non-depolarizing neuromuscular blocking drugs (nDNMBs)) in plasma when administered IV.
- Decreases concentration of functional nDNMBs in plasma, creating a concentration gradient from muscle tissue (high) to plasma (low).
- nDNMBs move from muscle compartment to plasma.
- Sugammadex in plasma encapsulates nDNMBs that moved to the plasma, decreasing concentration of functional nDNMBs in the plasma.
- Decreases concentration of nDNMBs at the nicotinic acetylcholine receptor within the skeletal neuromuscular junction.
- Reverses neuromuscular blockade created by nDNMBs.
Effects
- Movement of limbs or body during anesthesia.
- Coughing during anesthesia.
- Grimacing or suckling on the endotracheal tube.
Side Effects
-
Unknown mechanisms:
- Post-operative nausea and vomiting.
- Headache.
- Bradycardia.
- Cardiovascular collapse.
-
Progesterone is similar in structure to nDNMBs.
- Sugammadex binds progesterone, decreasing progesterone activity in the body.
- Progesterone is critical for maintenance of early pregnancy (unknown significance, avoid use in early pregnancy).
- Decreases effectiveness of progesterone-based contraception for 7 days.
-
Clearance in patients with severe renal impairment.
-
Binds to IgG or IgE receptors on sensitized basophils/mast cells in allergic reactions.
- Activation of basophils/mast cells.
- Degranulation of basophils/mast cells.
- Release of granulation products.
- Results in anaphylaxis, bronchospasm, hypotension.
Dosing
- 16mg/kg = Immediately post RSI dose of Roc
- 2-4mg/kg can reverse low Roc doses 0.6mg/kg in 3 minutes
- Depth of block and quantitative measurement for neostigmine and sugammadex:
| Depth of Block | Quantitative Measurement | Neostigmine (µg/kg) | Sugammadex (mg/kg) |
|---|---|---|---|
| Complete block | PTC = 0 | Not effective | 16 |
| Deep block | PTC ≥1 | Not effective | 4 |
| Moderate block | TOFC = 1-3 | Not effective | 2 |
| Shallow block | TOFC = 4, TOFR <0.2 | 50-70 | 1*-2 |
| Shallow/minimal block | TOFR: 0.2-0.5 | 40 | 0.75*-2 |
| Minimal block | TOFR: 0.5-0.7 | 20 | 0.25*-2 |
| Minimal block | TOFR: 0.7-0.9 | 10 | 0.25*-2 |
Contra-indications
- Allergy
- Renally impairment (CrCl <30)
- Bleeding or coagulopathic (prolongs APTT and INR)
- Pregnancy and breastfeeding
Benzodiazepines
Mechanism of Action
- Binds to Gamma-aminobutyric acid (GABA_A) receptor in vascular smooth muscle and the central nervous system (CNS).
- Increases frequency of chloride channel opening.
- Hyperpolarization of nerve membrane.
- Inhibits action potential (AP).
Effects
-
Vascular Smooth Muscle:
- APs inhibited.
- Vascular smooth muscle relaxes.
- Vasodilation.
- Decreases blood pressure, resulting in hypotension.
- Decreases cerebral blood flow and intracranial pressure.
-
Medulla Oblongata (Respiratory Center):
- APs inhibited.
- Decreases respiratory drive.
- Increases arterial CO2, resulting in hypercapnia.
- Decreases PaO2, resulting in hypoxemia.
- Pharyngeal muscle relaxation, leading to airway obstruction and temporary cessation of breathing (apnea).
-
CNS:
- General inhibition.
- Anti-convulsion effect (seizures caused by uncontrollable, electrical activity in the brain).
- Decreases anxiety, resulting in anxiolysis.
- In high doses, causes depression of arousal and loss of consciousness, leading to induction of general anesthesia (no analgesic effect).
-
Thalamus and Hypothalamus:
- APs inhibited.
- Causes amnesia.
-
Limbic System (Behavioral and Emotional Response Centers):
- APs inhibited.
Benzodiazepine Reversal
- Flumazenil:
- Competitively binds to GABA_A.
- Reverses the binding of benzodiazepine to GABA_A.
- Decreases frequency of chloride channel opening.
- Depolarization of nerve membrane.
Benefits of Alternatives
- Remimidazolam:
- Shorter acting
- Organ independent metabolism
- Fosproprofol:
- Prodrug
- Overcomes disadvantages of lipid based formulations
Local Anaesthetics
Local Anesthetics (Lidocaine, Bupivacaine, Ropivacaine, Procaine, Cocaine)
Mechanism of Action and Pharmacodynamics
Classification
- Ester Class:
- Cocaine, Procaine.
- Amide Class:
- Lidocaine, Ropivacaine, Bupivacaine.
Addition of a Vasoconstrictor (e.g., Epinephrine)
- Local vasoconstriction at the site of anesthetic infiltration.
- Decreases systemic absorption of the anesthetic.
- Increases anesthesia duration.
- Decreases bleeding at the site of infiltration.
- Larger amounts of local anesthetic can be safely administered.
pH Adjustment
- Increasing pH of injected solution (e.g., adding sodium bicarbonate).
- Decreases pKa of local anesthetic.
- Increases unprotonated form of local anesthetic.
- Increases uptake of anesthetic into neurons.
- Shortens onset time.
Local Infiltration
- Diffusion of the drug into tissue.
- When the tertiary amine of the anesthetic is unprotonated, it is lipophilic and can diffuse across nerve cell lipid membranes.
- Anesthetic molecule becomes protonated within the neuron.
- Protonated anesthetic binds to the transmembrane sodium channel.
- Sodium channels become impermeable to sodium ions, preventing movement across the membrane.
- Decreases nociceptive neuronal depolarization.
- Decreases pain sensation.
Structural Traits
- Aliphatic side chains and lipophilic linkages.
- High molecular weight.
- High lipophilicity.
- Increases anesthetic diffusion through nerve sheaths.
- Increases anesthetic potency.
Differential Nerve Blockade
- Anesthetics’ effect differs based on neuronal features.
- Small diameter neurons (e.g., unmyelinated nociceptive neurons) have fewer sodium channels for anesthetic to inhibit, thus blocking pain sensation more quickly than motor function.
- Unmyelinated neurons allow anesthetic to diffuse freely anywhere along the neuron.
Side Effects and Complications of Local Anesthetics
Classification
- Ester Class:
- Cocaine, Procaine.
- Amide Class:
- Ropivacaine, Bupivacaine, Lidocaine
Molecular Structure
- Both classes contain a lipophilic aromatic ring and a hydrophilic tertiary amine, making them amphipathic molecules.
Metabolism
- Ester Local Anesthetics:
- Metabolized by plasma cholinesterases.
- Impaired breakdown in patients with atypical pseudocholinesterase or pseudocholinesterase deficiency, leading to prolonged clearance and increased duration of anesthetic blockade.
- Amide Local Anesthetics:
- Metabolized in the liver by hepatic enzymes.
- Impaired breakdown in patients with liver disease, leading to prolonged clearance and increased duration of anesthetic blockade
Local Infiltration
- Diffuses into sympathetic nerves, blocking sodium channels on neuronal membranes and inhibiting sympathetic nerve function.
- Decreases vasoconstrictive signals to vascular smooth muscle.
- Causes vasodilation of systemic arteries, leading to hypotension.
- Some local anesthetic may enter systemic circulation and reach the heart, blocking ion channels on cardiac myocytes.
- If enough anesthetic reaches cardiac myocytes, their action decreases myocardial electrical signaling and thus decreases cardiac rhythm and contractility.
- Can result in cardiac arrest.
Bupivacaine Vs Ropivacaine
Common Characteristics
- pKa: Both bupivacaine and ropivacaine have a pKa of 8.1.
- Preparation: Both are available in 0.5% preparations.
Ropivacaine
- Isomer: Ropivacaine is the L-isomer.
- Lipid Solubility: Reduced lipid solubility compared to bupivacaine.
- Potency: Lower potency due to reduced lipid solubility.
- Vasoconstriction: Acts as a vasoconstrictor at lower doses.
- Cardiotoxicity: Less cardiotoxic due to lower lipid solubility.
- Motor Block: Results in less motor block.
- Concentration/Dose: Higher concentrations or doses are often required compared to bupivacaine for equivalent effect.
Bupivacaine
- Lipid Solubility: Higher lipid solubility compared to ropivacaine.
- Potency: Higher potency due to increased lipid solubility.
- Cardiotoxicity: More cardiotoxic due to higher lipid solubility.
- Motor Block: Results in more pronounced motor block.
Dantrolene
Mechanism of Action and Adverse Side Effects
Mechanism of Action
- Binds to ryanodine receptors (RYR1) in the sarcoplasmic reticulum of skeletal muscle cells.
- Prevents ryanodine channel from opening when triggered by the action potential in the muscle.
- Prevents calcium release from the sarcoplasmic reticulum.
- Prevents binding of calcium to troponin on the actin filaments in the cytosol of skeletal muscle cells.
- Myosin-binding site on the actin remains covered by tropomyosin.
- Prevents cross-bridge formation between myosin and actin within the sarcomere.
- Prevents muscle contraction.
- Results in decreased muscle rigidity and spasms.
- Decreases heat produced by muscular contraction, lowering body temperature.
- Skeletal and respiratory muscle weakness decreases inspiratory capacity, leading to dyspnea.
Adverse Side Effects
- Metabolized in the liver by the cytochrome P450 enzyme.
- Metabolic process forms a high concentration of hydroxylamine.
- Hydroxylamine is a highly toxic metabolite associated with dantrolene-induced liver injury, leading to impaired liver function.
Opioids
Opioid Receptor Agonists
Mechanism of Action
- Binds to opioid receptor subtypes in nervous tissues (mu (μ), kappa (κ), delta (δ)).
- Activation of G-protein coupled receptors.
- Inhibits adenylyl cyclase.
- Decreases conductance of presynaptic Ca²⁺ channels.
- Increases conductance of postsynaptic K⁺ channels.
- Inhibits postsynaptic neuron, decreasing neurotransmitter release.
- Inhibits pain transmission to the brain.
Effects
-
Central Actions:
- μ₁-receptor agonism in the brain and spinal cord.
- Release of pain NTs (e.g., glutamate, substance P, CGRP) from nerve fibers.
- GABA-mediated inhibition of dopamine release.
- Increases innervation of the oculomotor nerve.
- Results in analgesia, euphoria, miosis (pupillary constriction), urinary retention, slow and irregular respiratory rhythm, bradycardia, venous pooling, hypotension, pruritus, urticaria, bronchospasm, nausea/vomiting, and constipation.
-
Peripheral Actions:
- Direct stimulation of mast cells, resulting in mast cell degranulation and histamine release.
- Direct stimulation of the CTZ.
- μ₂-receptor agonism in the ENS, decreasing propulsive peristalsis and increasing delayed GI transit, leading to increased fluid reabsorption and constipation.
Mechanisms of Opioid Analgesia in the Peripheral Nervous System
Mechanism of Action
-
Exogenous Opioids:
- Derived from an external source (e.g., morphine, heroin, fentanyl).
-
Endogenous Opioids:
- Produced naturally in the body.
- Released in response to or in anticipation of painful stimuli (endorphins, enkephalins, dynorphins).
Effects
- Activation of opioid receptor subtypes (μ, κ, δ) in nervous tissues.
- Inhibits adenylyl cyclase.
- Decreases conductance of presynaptic Ca²⁺ channels.
- Increases conductance of postsynaptic K⁺ channels.
- Inhibits postsynaptic neuron, decreasing neurotransmitter release.
- Decreases pain transmission to the brain.
- Inhibition of GABA-releasing neurons in the periaqueductal grey (PAG) area.
- Activation of descending inhibitory pathways from PAG.
- Inhibits peripheral nociceptive signaling to the thalamus and other brain areas, resulting in inhibition of pain transmission to the brain.
Systemic Side Effects
- Confusion, delirium, addiction, myoclonus, miosis, sedation, somnolence, respiratory depression, nausea/vomiting, constipation, xerostomia (dry mouth), urinary retention, increased serum prolactin, increased sweating.
NSAIDS
Mechanism of Action
- Nonselective cyclooxygenase 1 and 2 inhibitors.
- Competitive or non-competitive antagonism of cyclooxygenase 1 or 2, impairing metabolism of arachidonic acid.
- Decreases production of thromboxane A2, resulting in decreased platelet aggregation and primary coagulation, increasing bleeding risk.
Effects
-
Central Actions:
- Decreases production of PGD, PGE, PGF, PGI.
- Decreases bronchoconstriction, resulting in bronchodilation and bronchospasm.
- Decreases PG-mediated renin release, leading to hyperkalemia.
- Constriction of afferent renal arterioles, decreasing glomerular filtration rate, increasing BP and fluid retention.
- Inhibits uterine contraction, decreasing uterine contractility and delaying labor.
-
Peripheral Actions:
- Decreases PG-mediated sensitization of pain receptors, resulting in analgesia and anti-inflammatory effects.
- Decreases fatty acid metabolism (oxidative phosphorylation) in the liver, leading to waste accumulation and hepatotoxicity.
- Impairs activity of outer hair cells and affects peripheral and central auditory neurons, resulting in ototoxicity and tinnitus.
- Irritation to stomach mucosa and impaired repair of stomach lining, leading to gastric ulcers (COX 2 selectivity decreases risk of ulcer).
- Decreases platelet aggregation, resulting in decreased primary coagulation and increased bleeding risk.
Quick Facts
- Primary Indication: Inflammatory disease, analgesia.
- Route(s) of Administration: Parenteral, oral & rectal.
- Metabolism: Primarily hepatic.
- Excretion: Primarily renal.
- COX 1 is in all cell types.
- COX 2 is up-regulated in inflammatory processes.
Arachidonic Acid Pathway
Mechanism of Action
- Membrane lipid (e.g., phosphatidylinositol) → phospholipase A2 → arachidonic acid.
- Lipoxygenase Pathway:
- Leukotrienes (LTB4, LTC4, LTD4, LTE4), resulting in neutrophil chemotaxis and bronchoconstriction.
- Cyclooxygenase Pathway:
- COX-1, COX-2 → endoperoxides (PGG2, PGH2) and thromboxane (TXA2), resulting in platelet aggregation and vasoconstriction.
- Prostacyclin (PGI2), resulting in decreased platelet aggregation, vasodilation, and decreased uterine tone.
- Prostaglandins (PGE2), resulting in decreased vascular tone, pain, decreased uterine tone, and decreased temperature.
- Lipoxygenase Pathway:
- Inhibitors:
- Corticosteroids inhibit phospholipase A2 and protein synthesis.
- NSAIDs, acetaminophen, COX-2 inhibitors, aspirin inhibit cyclooxygenase.
- Zileuton inhibits lipoxygenase.
- Zafirlukast, montelukast inhibit leukotriene receptors.
Cyclooxygenase (COX) 1 Vs COX 2
COX 1
- Function:
- Housekeeping enzyme.
- Protects stomach mucosa.
- Regulates kidney perfusion.
- Facilitates platelet aggregation.
- Production:
- Continuously produced (constitutive).
COX 2
- Function:
- Inflammatory, inducible enzyme.
- Advantages and Disadvantages:
- Benefits:
- Lower bleeding risk.
- Less gastrointestinal (GI) toxicity.
- Side Effects:
- Thrombotic complications.
- Cardiovascular (CV) events.
- Worsens hypertension (more than non-selective NSAIDs).
- Contraindicated in patients with sulfur drug allergies (e.g., celecoxib, paracoxib).
- Potential to cause renal dysfunction.
- Benefits:
Side Effects of NSAIDs
- Gastrointestinal:
- Gastric irritation.
- Coagulation:
- Contraindicated in neurosurgery and with warfarin use.
- Renal:
- Prostaglandins regulate renal blood flow and inhibit antidiuretic hormone (ADH).
- Respiratory:
- Bronchospasm and asthma (increased activity of the lipo-oxygenase pathway).
- Central Nervous System (CNS):
- Dizziness, vertigo, headaches, insomnia.
- Cardiovascular System (CVS):
- Fluid retention.
- Interferes with blood pressure medications.
- Closes patent ductus arteriosus (PDA).
- Skeletal:
- May inhibit osteoclasts.
- Thrombotic Effects:
- Increased risk of myocardial infarction (MI) and stroke.
- Drug Interactions:
- Displaces lithium and warfarin from plasma proteins.
- Hepatic:
- Hepatotoxicity (idiosyncratic).
Paracetamol
Dosing Guideline
- Standard Dose for Adults Over 50 kg:
- 1 g up to four times a day.
- Minimum 4 hours between each administration.
- 6 hours between doses for those with renal impairment (creatinine clearance 30 ml/min).
- Weight-Adjusted Dosing:
- Not endorsed despite high lipid solubility and low protein binding.
- Suggested regimen based on pharmacokinetic data: Single loading dose of 2 g, followed by 1 g doses every 4–6 hours.
- Postoperative Analgesia:
- Studies show lower pain scores and greater duration of effective pain relief with a 2 g loading dose compared to 1 g, with no increase in side effects or toxicity markers.
Pharmacokinetics
- Administration:
- Oral: Tablets, capsules, syrup.
- Rectal.
- IV: Perfalgan®.
- Bioavailability:
- Oral: 80%.
- IV: 100% bioavailability with predictable onset of action (OOA) within 15 minutes and peak effect within 1 hour.
- Oral and rectal routes have lower bioavailability and unpredictable absorption and OOA.
- Peak Plasma Levels After Administration:
- Tablets: 1 – 2 hours.
- Syrup: 30 minutes.
- IV: 15 minutes after infusion completed.
- Elimination Half-life (t₁/₂):
- 1.5 – 3 hours.
- Volume of Distribution:
- 0.9 L/kg.
- Protein Binding:
- 10%.
- Metabolism:
- Hepatic.
- Excretion:
- Renal, sulfate, and glucuronide metabolites.
Mechanism of Action
-
Prostaglandin Inhibition:
- Inhibits prostaglandin H₂ synthetase at the cyclooxygenase (COX) and peroxidase (POX) sites.
- Reduces the conversion of arachidonic acid to prostaglandin G₂.
- Inhibits the subsequent conversion of prostaglandin G₂ to prostaglandin H₂.
- This reduces the levels of downstream prostaglandins (PGE₂, PGI₂, TXA₂).
-
Endocannabinoid Reuptake Inhibition:
- Metabolized to p-aminophenol.
- p-Aminophenol is converted to AM404 in the brain.
- AM404 inhibits the reuptake of anandamide, an endogenous cannabinoid, contributing to analgesic effects.
-
Serotonergic Pathway:
- Involves descending pain pathways from brainstem nuclei, hypothalamus, and cortex interacting with pain afferents in the dorsal horn.
- Serotonin receptors, especially 5-HT3, are involved in consciousness, mood, memory, and nausea/vomiting.
- Activation of descending serotonergic pathways is key to paracetamol’s action.
- Antinociceptive effects can be partially inhibited by co-administration of 5-HT3-receptor antagonists, frequently given with paracetamol perioperatively.
-
Endocannabinoid Enhancement:
- Paracetamol (via p-aminophenol) conjugates with arachidonic acid to form AM404.
- AM404 inhibits anandamide reuptake, increasing cannabinoid receptor activation.
- Contributes to relaxation, tranquility, and euphoria, independent of analgesia.
- AM404 also activates TRPV1, and inhibits COX, NO, and TNF-alpha, all involved in pain states.
- Central production of AM404 accounts for antipyretic effects related to inhibition of brain prostaglandin production.
Statins
MOA
Cholesterol-Lowering Effects
- Statins are effective in lowering cholesterol levels in patients.
Pleiotropic Effects
- Improved Endothelial Function:
- Enhances the function of the endothelium, the inner lining of blood vessels.
- Altered Expression of Nitric Oxide Synthase (NOS):
- Modifies the expression of NOS, which is crucial for the production of nitric oxide, a molecule that helps relax and dilate blood vessels.
- Inhibits Thrombogenic Response:
- Reduces the body’s tendency to form blood clots.
- Platelet Inhibition:
- Inhibits platelet aggregation, decreasing the risk of clot formation.
- Reduced Vascular Inflammation:
- Lowers inflammation within the blood vessels, which can contribute to atherosclerosis and other cardiovascular diseases.
- Stabilization of Atherosclerotic Plaques:
- Helps stabilize plaques in the arteries, reducing the risk of plaque rupture and subsequent cardiovascular events.
- Reduction in Major Adverse Cardiovascular Events (MACE):
- Statins have been associated with a 19% reduction in the incidence of major adverse cardiovascular events.
Guidelines and Recommendations
- ACC/AHA and ESC/ESA Guidelines:
- Perioperative Continuation of Statins:
- Strongly recommend the continuation of statins during the perioperative period (Class 1 recommendation).
- Cessation of statins is associated with an increased risk of myocardial injury.
- Initiation of Statins for Vascular Surgery:
- Recommend the initiation of statins for patients undergoing vascular surgery (Class 2a recommendation).
- Perioperative Continuation of Statins:
Steroids
Equivalent Doses of Different Steroids
| Glucocorticoid | Equivalent Dose | Anti-inflammatory Activity (Relative to Hydrocortisone) | Duration of Action |
|---|---|---|---|
| Hydrocortisone (Cortisol) | 20 mg | 1 | 8-12 hours |
| Prednisolone/Prednisone | 5 mg | 4 | 12-36 hours |
| Methylprednisolone | 4 mg | 5 | 12-36 hours |
| Dexamethasone | 0.75 mg | 30 | 36-72 hours |
| Betamethasone | 0.6 mg | 30 | 36-72 hours |
Dexmedetomidine
Mechanism of Action
- Arousal Effects:
- Acts on presynaptic α2 adrenergic receptors projecting from the locus ceruleus.
- Binds to α2 receptors, hyperpolarizing locus ceruleus neurons, decreasing norepinephrine release.
- Burst Suppression:
- Rarely associated with burst suppression (a profound state of anesthetic-induced brain inactivation) common with high doses of propofol, barbiturates, or inhaled ether drugs.
- Sedation:
- Provides sedation with minimal risk of respiratory depression.
- Commonly used as an anesthetic adjunct in the operating room due to easy arousability of patients.
- Procedural sedation associated EEG pattern resembles nonrapid eye movement (NREM) sleep stage III or slow-wave sleep, characterized by slow/delta oscillations.
- Detailed MOA:
- Blocks norepinephrine (NE) release from neurons projecting from the locus ceruleus (LC) to various brain areas:
- Preoptic area (POA) of the hypothalamus.
- Basal forebrain (BF).
- Intralaminar nucleus (ILN) of the thalamus.
- Cortex.
- Hyperpolarization of locus ceruleus neurons results in loss of inhibitory inputs to the preoptic area of the hypothalamus, activating inhibitory pathways from the preoptic area to arousal centers, leading to decreased arousal.
- Loss of excitatory inputs from the locus ceruleus to the basal forebrain, intralaminar nucleus of the thalamus, and cortex decreases thalamocortical connectivity, enhancing the sedative effect.
- Blocks norepinephrine (NE) release from neurons projecting from the locus ceruleus (LC) to various brain areas:
SASSA Recommendations for Use
- Does not support any specified limitations.
- Encourages responsible, sparing use in patients who will specifically benefit.
- Dose should be guided by Neuromuscular Transmission (NMT) monitoring.
- Does not encourage routine use due to unsustainability in South Africa.
- Advocates the use of Neo/AC for the majority of patients.
- Continued practice in the patient’s best interest, both clinically and financially.
- Recommends ready availability at all facilities.
Dexmedetomidine as an Adjuvant in Neuraxial and Peripheral Regional Anesthesia: Updated Evidence and Guidelines (2020–2025)
Mechanism of Action
- α2-Adrenergic Agonism: Dexmedetomidine (DEX) exhibits high selectivity for α2 over α1 receptors (α2:α1 ≈ 8–10:1), leading to sedation, analgesia, and sympatholysis.
- Hyperpolarization via HCN Channel Blockade: DEX inhibits hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, enhancing analgesia and potentiating local anesthetic effects.
- Analgesia: Mediated through inhibition of substance P and glutamate release in the dorsal horn of the spinal cord.
- Sedation: Achieved by activation of α2 receptors in the locus coeruleus
- Vasoconstriction: Mild α1 activity may delay systemic uptake of local anesthetics, prolonging their effect.
General Safety
- Regulatory Status: DEX is not FDA-approved for obstetric use or non-intravenous routes.
- Pregnancy Category: Classified as Category C; crosses the placenta but results in minimal fetal exposure.
- Neonatal Outcomes: Studies report no significant changes in fetal heart rate, Apgar scores, or neonatal outcomes.
- Neurotoxicity: No reported neurotoxic effects with neuraxial or perineural administration
Clinical Applications and Dosing by Route
1. Intravenous (IV) Use in Obstetric Anesthesia
- Indications: Intraoperative sedation, analgesia, shivering control, and potential PTSD prevention.
- Dosing:
- Sedation: Continuous infusion at 0.2–0.5 µg/kg/hr.
- Shivering: Single bolus of 0.5 µg/kg.
- Benefits:
- Minimal respiratory depression.
- Reduced intraoperative pain in cesarean section patients.
- Effective shivering control compared to alternatives like meperidine.
- Potential reduction in PTSD incidence post-cesarean section.
2. Intrathecal Dexmedetomidine
- Proposed Mixture:
- DEX: 2–4 µg (based on 4 µg/mL preservative-free vial).
- Local Anesthetic: 10–13.5 mg of 0.75% hyperbaric bupivacaine.
- Optional: Intrathecal morphine for extended analgesia.
- Outcomes:
- Faster onset and prolonged duration of sensory and motor block.
- Reduced postoperative pain and shiveriring
- Decreased need for intraoperative opioids.
- Potential for hypotension and bradycardia; preparedness with vasopressors and anticholinergics recommended.
- Evidence
- A study determined the ED50 of intrathecal DEX as an adjuvant to plain ropivacaine for spinal anesthesia during cesarean section to be approximately 5.9 µg with 8 mg ropivacaine and 3.1 µg with 10 mg ropivacaine.
3. Epidural Dexmedetomidine
A. Epidural Conversion for Cesarean Section
- Mixture: 4 µg DEX added to 10–20 mL of 2% lidocaine, with or without bicarbonate.
- Outcome: Faster onset and improved intraoperative analgesia.
B. Epidural Top-Ups (Second Stage Labor)
- Mixture: 2–4 µg DEX added to standard bolus (e.g., 4 mL of 0.1% ropivacaine with fentanyl or 2–3 mL of 0.2% ropivacaine).
- Benefit: Enhanced assessment of block effectiveness and improved analgesia.
C. Continuous Labor Epidural Infusions
- Mixture: 0.1% ropivacaine with 0.3–0.5 µg/mL DEX.
- Outcomes:
- Reduced local anesthetic consumption.
- Decreased patient-controlled epidural analgesia (PCEA) boluses.
- Lower incidence of pruritus and nausea.
- No significant maternal or neonatal side effects.
- Optimal concentration identified as 0.3 µg/mL to minimize motor block.
- Evidence:
- A study found that dexmedetomidine combined with local anesthetic for epidural labor analgesia improved pain scores without prolonging labor stages
4. Peripheral Nerve Blocks
- Mixtures:
- Outpatient Procedures: 0.5–1 µg/kg DEX with local anesthetic.
- Inpatient Procedures: 1–2 µg/kg DEX with local anesthetic.
- Benefits:
- Shortened onset time of anesthesia.
- Prolonged analgesia by approximately 3–4 hours.
- Opioid-sparing effect.
- Cautions:
- Transient hypotension observed at higher doses (1.5–2 µg/kg).
- Contraindicated in patients with bradycardia, hypotension, pulmonary hypertension, obstructive sleep apnea, frailty, or shock.
- Key Studies
- A meta-analysis revealed that perineural DEX doses of 30–50 µg are appropriate, providing prolonged analgesia without increasing the risk of bradycardia and hypotension
5. Nebulized Dexmedetomidine for Post-Dural Puncture Headache (PDPH)
- Dose: 1 µg/kg diluted to 4 mL with saline, administered every 12 hours.
- Benefits:
- Reduction in cerebral blood flow, alleviating PDPH symptoms.
- Increased cerebrospinal fluid (CSF) pressure due to decreased absorption.
- Non-invasive and opioid-sparing approach.
- Minimal hemodynamic or sedative effects.
Dexmedetomidine as an Adjuvant in Regional Anesthesia
| Route / Application | Dose / Concentration | Evidence Summary | Benefits | Comparisons / Notes |
|---|---|---|---|---|
| Intrathecal (CS, ortho) | 2–4 µg+ 10–13.5 mg 0.75% bupivacaine ± morphine | Liu et al. 2020; Mo et al. 2023: prolonged block (~2h), reduced pain, ED50 ≈ 3.1–5.9 µg | Fast onset, dense and prolonged block, ↓ shivering, ↓ opioid need | Superior to fentanyl for block duration and shivering reduction |
| Epidural Conversion (CS) | 4 µg + 10–20 mL 2% lidocaine ± bicarbonate | Riham et al. 2016: improved onset, analgesia vs epinephrine | Rapid CS conversion, better intra-op analgesia | Comparable or better than epinephrine |
| Epidural Top-Up (2nd Stage) | 2–4 µg + 4 mL 0.1% ropivacaine ± fentanyl OR 2–3 mL 0.2% ropivacaine | Qian et al. 2021; Yang et al. 2020: faster onset, less nausea/shivering vs fentanyl | Improved block efficacy, less shivering and nausea | Faster onset and fewer side effects than fentanyl |
| Continuous Epidural Infusion | 0.3–0.5 µg/mL in 0.1% ropivacaine | Wei et al. 2022; Pang et al. 2022: fewer PCEA boluses, lower LA use, optimal dose = 0.3 µg/mL | Longer duration, fewer PCEA uses, less pruritus | Better tolerability than sufentanil |
| Peripheral NB (Outpatient) | 0.5–1 µg/kg + local anesthetic | Dai et al. 2018: prolonged block (~3–4h), better analgesia | Faster onset, prolonged analgesia, opioid-sparing | ↑ bradycardia/hypotension vs ropivacaine alone |
| Peripheral NB (Inpatient) | 1–2 µg/kg + local anesthetic | Packiasabapathy et al. 2017; Jung et al. 2018: 2 µg/kg superior to 1 µg/kg for duration (~20h) | Longest block duration (~20h), ↓ postop opioid use | 2 µg/kg better than 1 µg/kg; ↑ hypotension risk |
| Nebulized (PDPH) | 1 µg/kg diluted to 4 mL saline, q12h | Kumar et al. 2019; Mowafy et al. 2021: ↓ PDPH severity, increased CSF pressure, minimal side effects | Non-invasive PDPH relief, minimal sedation | Alternative to conservative PDPH management |
Albumin
Manufacturing
- Process:
- Produced through the Cohn cold ethanol fractionation process.
- Resulting in a sterile aqueous solution of albumin obtained from large pools of adult human venous plasma.
Functions
-
Binding and Transport:
- Binds and transports drugs and endogenous molecules, such as bilirubin, fatty acids, bile salts, steroids, and acidic drugs (e.g., Warfarin, aspirin, furosemide, amiodarone).
-
Oncotic Pressure:
- Accounts for 80% of intravascular oncotic pressure.
-
Free Radical Scavenging:
- Acts as a scavenger for free radicals.
-
Acid-Base Balance:
- Contributes significantly to the anion gap.
-
Calcium Handling:
- Negative charge of albumin binds Ca²⁺, reducing free Ca²⁺ levels.
Interactions
- Protein Binding:
- Binding of acidic drugs to albumin reduces free drug concentration and increases the volume of distribution (Vd).
- Hypoalbuminaemia increases free drug levels, while albumin infusions decrease free drug levels.
- Subject to competition, where one drug’s protein binding may reduce another’s due to higher affinity, increasing free drug concentration of the latter.
- Non-competitive allosteric interactions are possible, where one drug causes a conformational change in albumin, displacing another drug.
- Examples:
- Warfarin, aspirin, furosemide, amiodarone.
Disadvantages
- Expensive.
- Greater need for ventilatory support.
- Negative inotropic effects.
- No ability to decrease cascade activation.
- No data showing reduced morbidity/mortality.
Contraindications
- Known allergy.
- Cardiac failure.
- Pulmonary edema.
- Severe anemia.
Links
References:
- Image: Novice Anaesthesia. (2021). Infographics. Retrieved April 24, 2025, from https://www.gasnovice.com/infographics
-
- Bao, N., Shi, K., Wu, Y. et al. Dexmedetomidine prolongs the duration of local anesthetics when used as an adjuvant through both perineural and systemic mechanisms: a prospective randomized double-blinded trial. BMC Anesthesiol 22, 176 (2022).(https://doi.org/10.1186/s12871-022-01716-3)
- Talke P, Anderson BJ. Pharmacokinetics and pharmacodynamics of dexmedetomidine-induced vasoconstriction in healthy volunteers. Br J Clin Pharmacol. 2018 Jun;84(6):1364-1372. doi: 10.1111/bcp.13571. Epub 2018 Apr 2. PMID: 29495085; PMCID: PMC5980451.(https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.13571)
- Sween LK, Xu S, Li C, O’Donoghue MA, Ciampa EJ, Kowalczyk JJ, Li Y, Hess PE. Low-dose intravenous dexmedetomidine reduces shivering following cesarean delivery: a randomized controlled trial. Int J Obstet Anesth. 2021 Feb;45:49-55. doi: 10.1016/j.ijoa.2020.11.004. Epub 2020 Nov 17. PMID: 33293185. (https://pubmed.ncbi.nlm.nih.gov/33293185/)
- Kang H, Lim T, Lee HJ, Kim TW, Kim W, Chang HW. Comparison of the effect of dexmedetomidine and midazolam under spinal anesthesia for cesarean delivery: a randomized controlled trial, single center study in South Korea. Anesth Pain Med (Seoul). 2023 Apr;18(2):159-168. doi: 10.17085/apm.22257. Epub 2023 Apr 28. PMID: 37183284; PMCID: PMC10183612 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10183612/).
- Liu S, Zhao P, Cui Y, Lu C, Ji M, Liu W, Jiang W, Zhu Z, Sun Q. Effect of 5-μg Dose of Dexmedetomidine in Combination With Intrathecal Bupivacaine on Spinal Anesthesia:(https://pubmed.ncbi.nlm.nih.gov/32222361/) A Systematic Review and Meta-analysis. Clin Ther. 2020 Apr;42(4):676-690.e5. doi: 10.1016/j.clinthera.2020.02.009. Epub 2020 Mar 25. PMID: 32222361.
- Khosravi F, Sharifi M, Jarineshin H. Comparative Study of Fentanyl vs Dexmedetomidine as Adjuvants to Intrathecal Bupivacaine in Cesarean Section: A Randomized, Double-Blind Clinical Trial. J Pain Res. 2020 Oct 7;13:2475-2482. doi: 10.2147/JPR.S265161. PMID: 33116789; PMCID: PMC7548853.(https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7548853/#:~:text=In%20the%20present%20study%20intrathecal,compared%20to%2025%20%CE%BCg%20fentanyl.)
- Richebé P, Brull SJ. Pharmacokinetics and pharmacodynamics of intravenous anaesthetics. Miller’s Anaesthesia. 10th ed. Elsevier; 2023.
- Westhoff J, Bailey PL. Context-sensitive half-time: relevance for anaesthetists. Br J Anaesth Educ. 2022;22:85-92. sciencedirect.com
- Deranged Physiology. Hepatic clearance and extraction ratio. 2024. derangedphysiology.com
- Minto CF, et al. Measured context-sensitive half-times of remifentanil and alfentanil. Anesthesiology. 1995;82:996-1005. pubmed.ncbi.nlm.nih.gov
- Polania Gutierrez JJ, Rocuts KR. Anaesthetic drug metabolism: current concepts. Anesth Analg. 2025;140:123-131. frontiersin.org
- Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 14th ed. McGraw-Hill; 2023.
- The Calgary Guide to Understanding Disease. (2024). Retrieved June 5, 2024, from https://calgaryguide.ucalgary.ca/
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org/
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
Summaries:
Physiology 01
Physiology 02
Autonomic NS
CVS
Bleeding and clotting
Antibiotics; Steroids; DM; Antipsychotics; Neuro
Inhalational agents
Local anaesthetics
NMB
Analgesics
NSAIDS
Opioids
Propofol
BZD
Anti-emetics
Anticonvulsants
Anti-cholinesterases
Antibiotics
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “6ea05e46-b248-4a99-934a-b43a52df811e”



