- Whipple's (Pancreatoduodenectomy)
- Gastrectomy
- Pancreas Transplant
- Links
{}
Whipple’s (Pancreatoduodenectomy)
Introduction
- Pancreatoduodenectomy, also known as Whipple’s procedure, may or may not be pylorus sparing and may or may not be duodenum sparing.
- The usual indication is to remove pancreatic cancers, but it may also be done for some benign pancreatic conditions, such as cysts, and for bile duct or duodenal cancers.
- Pancreatic cancer in general has a poor prognosis. It is often detected late, by which time it is unresectable with a 1-year survival rate under 10% and a 5-year survival rate less than 5%. It typically also spreads rapidly.
Procedure
- Usually performed via a subcostal incision or a longitudinal laparotomy incision, but some institutions offer a laparoscopic approach.
- Involves removal of the head of the pancreas, common bile duct, gall bladder, and sometimes part of the stomach and duodenum.
- A significant number of patients will prove to have unresectable disease, resulting in:
- No further action
- Simple bypass procedure
- Triple bypass procedure
- Decision-making on whether to proceed can be time-consuming and requires considerable dissection.
- The procedure typically lasts 4 to 12 hours, with an average of 6 hours, depending on technical difficulty.
- Pylorus sparing resection does not appear to provide any significant advantage or disadvantage.
- Rarely, the dissection may include a section of the portal vein, increasing complexity.
- Transplant surgeons are preferred for vascular repair over vascular surgeons due to their experience with portal vein anastomoses in liver transplants.
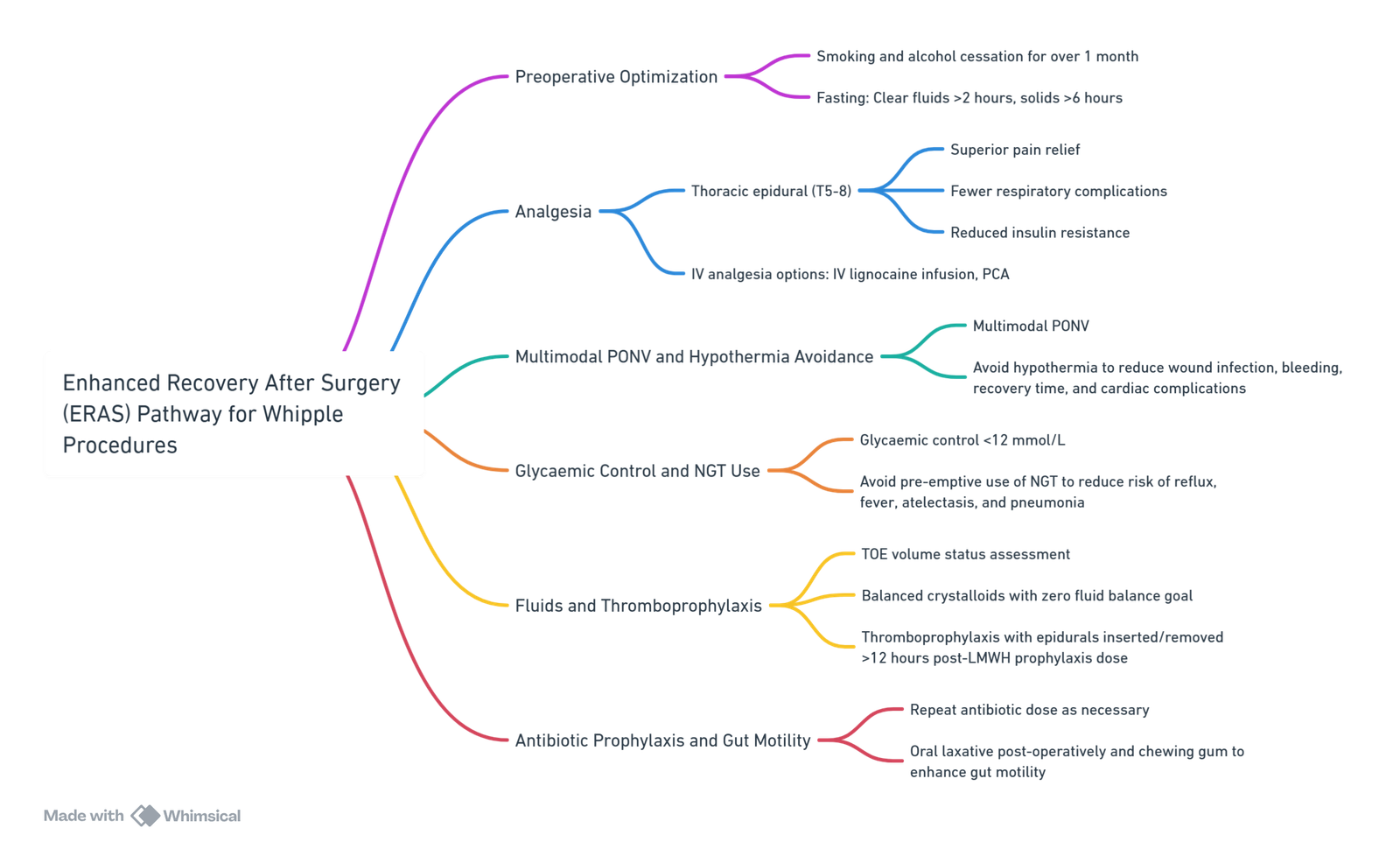
Conduct of Anaesthesia
View or edit this diagram in Whimsical.
- These procedures tend to be long and uneventful for most of the time, so preparation is essential.
- Listening to the surgical conversation during the initial hours of dissection can provide early insights into the resectability of the lesion.
- Most patients do not require a blood transfusion.
Pre-operative Considerations
- Common preoperative conditions include weight loss of 10-20%, jaundice, abdominal pain, and fatigue.
- Many jaundiced patients have had an endoscopic retrograde cholangio-pancreatography (ERCP) with biliary stent placement, resolving jaundice by the time of surgery.
- Preoperative drainage shows no harm or benefit if bilirubin levels are below 250 µmol/L.
- Pancreatitis or cholangitis may follow ERCP.
- Procedures should be done in centers performing over 20 per year for better perioperative care.
- ERCP is indicated pre-operatively when:
- Acute cholangitis is present (urgent decompression required).
- Severe, symptomatic jaundice is accompanied by pruritus, renal impairment, or coagulopathy.
- Bilirubin > 250 µmol/L and/or surgery will be delayed for logistical or medical reasons, making temporary biliary drainage desirable.
- There is diagnostic uncertainty requiring tissue diagnosis (e.g., brushings/biopsy during ERCP).
Premedication
- According to patient requirements. Usually, patients selected for resection are reasonably well.
- Low molecular weight heparin is omitted for the appropriate interval if an epidural is planned.
Optimization
- Many patients have a history of alcohol and smoking:
- Alcohol:
- Postoperative morbidity is increased two to threefold in alcohol abusers.
- One month of preoperative abstinence improves outcomes significantly.
- Smoking:
- Daily smokers (>2 cigarettes/day for >1 year) have an increased risk of pulmonary and wound complications.
- Reducing complications is possible with smoking cessation for 1 month.
- Alcohol:
Investigations
- Full blood count, urea, creatinine, electrolytes, ECG, chest radiograph, glucose, and lung functions for at-risk patients.
- Blood group and screen usually suffice unless antibodies are present—then cross-match two units.
- Ensure an ICU or high-care bed is available, with a preference for ICU for better post-op care.
Pre-induction
- Start with a good IV in case of blood loss (generally low incidence of transfusion).
- Epidural insertion at T8 for analgesia. Test dose given during transfer to the table if time-constrained.
- Standard monitoring (ECG, pulse oximeter, temperature) placed prior to induction.
Perioperative Considerations
Positioning
- Supine position as high as possible on the table for possible C-arm use.
- Arms tucked by the sides to prevent brachial plexus injuries.
- A screen at T4 level to prevent surgeons from leaning on the patient’s nose.
- A rotatable clamp allows the rail position to be adjusted as needed.
Induction and Maintenance
- Generally straightforward with preoxygenation, propofol, and a muscle relaxant of choice, followed by intubation.
- Laparoscopy may be performed beforehand on a separate occasion to check for metastases.
- Maintenance with air/oxygen, isoflurane or sevoflurane, and relaxant top-ups.
- Avoid nitrous oxide.
- Intravenous anaesthetic techniques are not advantageous.
- Arterial line placed after induction for better monitoring during long procedures.
- A nasogastric tube is not essential but may be needed for gastric outlet obstruction or stomach hyperinflation during induction.
Monitoring and Support
- Encourage surgeons to catheterize the bladder during induction.
- Place forced air warmer, temperature probe, and calf stimulators.
- Central venous catheters are used only for patients with poor venous access, significant cardiac disease, or specific indications.
- Fluid management aims at normovolaemia with a colloid/balanced salt mix.
- Epidural patients may require more fluid.
- Antibiotic prophylaxis typically with a cephalosporin.
Analgesia
- Epidural with 0.25% plain bupivacaine, usually 5-10 ml required for T4 Block. 3mg morphine loading optional
- Top-up with 3-5 ml bupivacaine hourly or run infusion at 80% of loading dose requirement (i.e if 10ml required for loading the run at 8ml/h)
- Avoid systemic fentanyl for better block assessment.
- Spinal morphine or IV morphine via patient-controlled systems or continuous infusions if epidural is not used.
- IV paracetamol near the end of surgery.
Emergence Phase
- Patients can generally be extubated after routine reversal of the relaxant.
- Severe systemic inflammatory response syndrome (SIRS) response may require ICU care and CVP line placement.
Post-operative Care
- Typically, 2 days in ICU or high care unit followed by about 10 days in the hospital if no complications arise.
Complications
In-Hospital
- Anastomotic leaks (10-15% incidence)
- New-onset diabetes (10% incidence)
- Infections, gastric emptying difficulties, and bleeding
- Short-term perioperative mortality: 4-15%
- Rapid growth of liver metastases within 6 weeks post-resection
Long-Term
- Continued weight loss
- Bloating, fullness, nausea, or vomiting
Nutritional Recommendation Before Surgery
Recommendation: Patients at risk (weight loss >10-15% within 6 months, BMI <18.5 kg/m², and serum albumin <30 g/l in the absence of liver or renal dysfunction) should receive oral nutritional supplements for 7 days prior to surgery. For severely malnourished patients (>10% weight loss), surgery should be postponed for at least 2 weeks to improve nutritional status and allow patients to gain weight.
Evidence Level: High
Grade of Recommendation: Strong
Gastrectomy
Introduction
Functions of the Stomach
- Storage and controlled delivery of chyme to the small bowel
- Mechanical and chemical digestion (acid, pepsin)
- Defence (gastric acidity limits bacterial load)
- Secretion (intrinsic factor, pepsinogens)
Risk Factors for Gastric Adenocarcinoma
- Age > 65 years is common
- Helicobacter pylori, smoking, atrophic gastritis, pernicious anaemia
- Prior gastric surgery (especially Billroth II)
- Genetic: e.g., CDH1 (E-cadherin) mutation → diffuse (linitis plastica) phenotype
- Low socioeconomic status and dietary factors (salted/smoked foods)
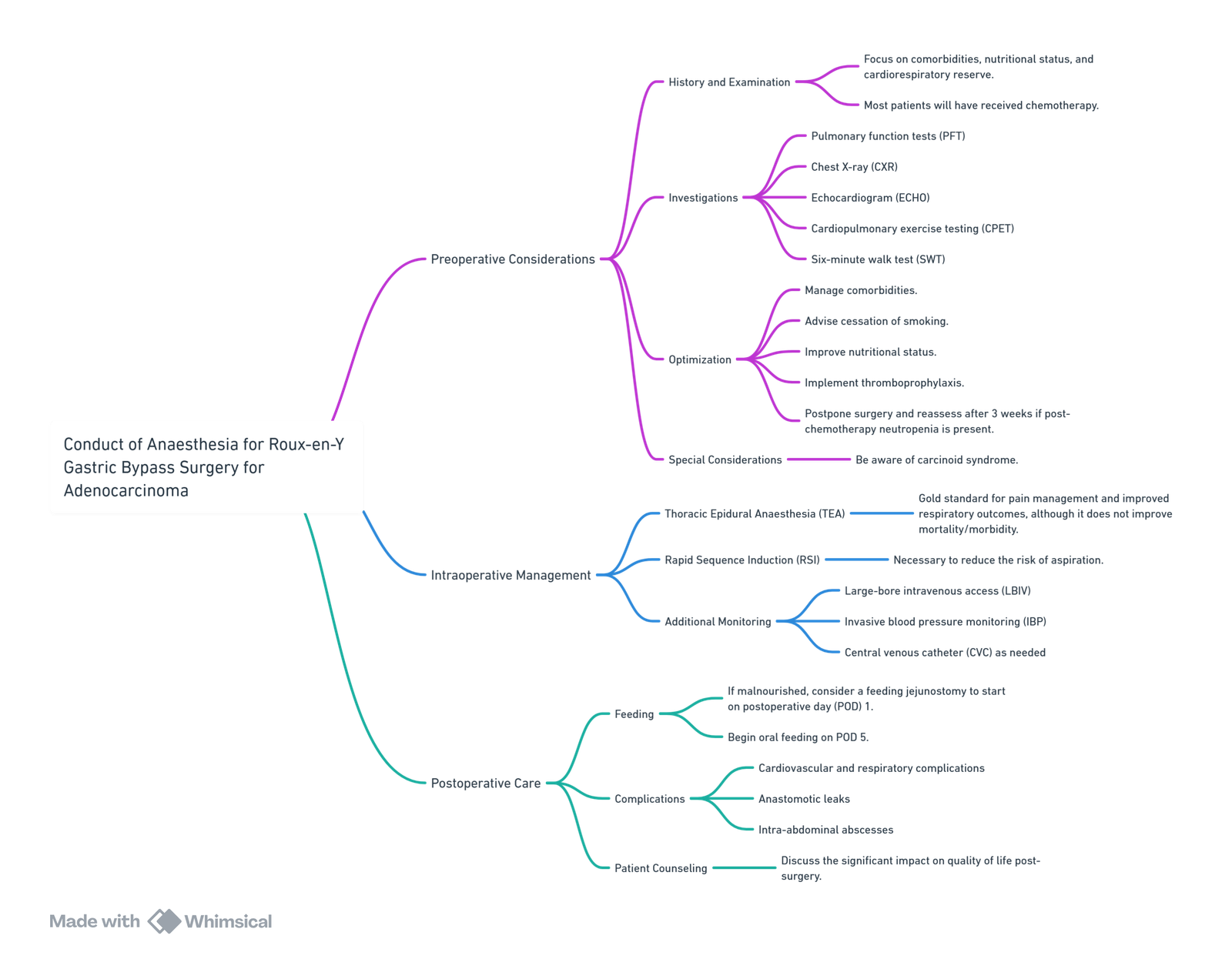
Anaesthesia for Gastrectomy with Roux-en-Y Reconstruction (gastric adenocarcinoma)
View or edit this diagram in Whimsical.
Pre-operative Assessment & Optimisation
History/examination
- Define disease stage, dysphagia/obstruction, weight loss/sarcopenia, reflux/aspiration risk, performance status and exercise tolerance.
- Document neoadjuvant therapy (many receive peri-operative FLOT: 5-FU, leucovorin, oxaliplatin, docetaxel) and toxicities: myelosuppression, neuropathy (oxaliplatin), and 5-FU-related coronary vasospasm (consider baseline ECG ± cardiology if symptoms).
Investigations (selective, not Routine Panel testing)
- FBC, U&E, LFTs; group and screen. Iron studies if anaemia.
- ECG; echocardiography if symptoms, murmurs, or cardiotoxic regimens/suspicion.
- Chest radiograph and pulmonary function tests only if indicated by symptoms/comorbidity.
- Consider cardiopulmonary exercise testing (CPET) in major upper-GI cancer surgery for objective risk stratification (e.g., AT < 11 ml kg⁻¹ min⁻¹ or V̇O₂peak < 15 ml kg⁻¹ min⁻¹ signals higher risk).
Effects of Neoadjuvant Chemotherapy
- Plan timing of surgery ~4–6 weeks after last cycle to allow marrow recovery; postpone if neutropenic (ANC < 1.0–1.5 ×10⁹ L⁻¹).
- Coordinate with oncology.
Nutrition & Pre-rehabilitation
- Screen for malnutrition/sarcopenia; refer early to dietetics. Use enteral route whenever feasible; immunonutrition can reduce infectious complications in gastric cancer surgery in some analyses.
- Carbohydrate loading (e.g., 800 mL night before + 400 mL 2–3 h pre-op) within ERAS is safe in non-obstructed patients; avoid in those with gastric outlet obstruction or high aspiration risk. Clear fluids permitted to 2 h pre-op per local policy.
- Smoking cessation ≥ 4 weeks pre-op reduces complications; provide pharmacotherapy + behavioural support.
VTE Prevention
- Cancer + major abdominal surgery = high risk. Use mechanical prophylaxis and commence pharmacological prophylaxis when haemostasis permits; extend LMWH to 28 days post-op.
Antimicrobial Prophylaxis
- Give first dose within 60 min before incision (120 min for vancomycin/fluoroquinolone), and re-dose per duration/blood loss; cefazolin ± metronidazole is typical per local microbiology.
Intra-operative Management
Airway & Aspiration
- Rapid sequence induction (RSI) for patients with symptomatic obstruction, severe reflux, or high aspiration risk; consider pre-operative NG decompression if obstructed.
Monitoring & Access
- Two large-bore IVs; arterial line for beat-to-beat BP and gases; central venous access only if strong indication (poor access, major fluid shifts). Active warming and urinary catheter.
Fluid Therapy
- Avoid overly restrictive strategies (↑ AKI/SSI in RELIEF). Use balanced crystalloids and goal-directed haemodynamic therapy in higher-risk cases.
Analgesia (tailor to Surgical approach)
- Open gastrectomy: Thoracic epidural analgesia (TEA, T6–T9) remains a strong option within ERAS for superior dynamic analgesia and reduced pulmonary complications vs IV opioids.
- Minimally invasive (laparoscopic/robotic): Epidural often not required; consider multimodal systemic analgesia plus regional techniques (e.g., bilateral TAP/QL, paravertebral or erector spinae plane blocks) according to local expertise. Recent trials show PCA-based regimens can be non-inferior to EDA here.
- NSAIDs: Use caution early after GI anastomosis; observational/meta-analytic data suggest non-selective NSAIDs (e.g., ketorolac) may be associated with higher anastomotic leak risk—prefer COX-2 selective agents if NSAID required. Balance with opioid-sparing benefits.
PONV Prevention
- Upper-GI surgery + opioids = high risk. Apply multimodal prophylaxis (≥ 2 classes; e.g., dexamethasone + 5-HT₃ antagonist ± NK1 antagonist; TIVA if feasible).
Post-operative Care (ERAS-aligned)
- Extubation in theatre if stable; HDU/ICU if high risk (poor CPET, major transfusion, extensive resection).
- Early oral intake (clear liquids on POD 0–1, advance as tolerated) and avoid routine NG decompression; early mobilisation and chest physio.
- Analgesia: Continue epidural if used; otherwise PCA ± regional catheter/blocks; regular paracetamol; consider COX-2 inhibitor where appropriate.
- VTE: continue pharmacological prophylaxis to 28 days
- Glycaemic control (stress hyperglycaemia ↑ SSI).
- Complications to anticipate: pulmonary complications, anastomotic leak, ileus, AKI, intra-abdominal collections. Prompt imaging/drainage where indicated.
Patients with prior Gastrectomy (or after Total gastrectomy)
- Expect and screen for deficiencies and neuropathy:
- Vitamin B₁₂: lifelong supplementation (e.g., 1 mg i.m. every 1–3 months or high-dose oral 1–1.5 mg day⁻¹) with periodic levels and clinical review.
- Iron (and ferritin), folate, vitamin D/calcium (bone health), ± thiamine if poor intake/protracted vomiting. Coordinate dietetics, and document any neurological deficits
Pancreas Transplant
Introduction
- In patients undergoing simultaneous pancreas and kidney transplantation (SPK), the transplanted kidney does not develop diabetic nephropathy.
- Improvements in retinopathy and neuropathies associated with type 1 diabetes have also been observed.
Types of Pancreas Transplantation
- Simultaneous Pancreas and Kidney Transplant (SPK)
- Pancreas After Kidney Transplant (PAK)
- Pancreas Alone Transplant (PAT)
- SPK is the most common procedure.
Patient Selection
- Primary determinants for recipient selection include the presence of diabetic complications, degree of nephropathy, and cardiovascular risk.
- Patients must have documented Type 1 diabetes, as there is no evidence to support transplantation in Type 2 diabetes.
Complications of Diabetes Mellitus
General:
- Infections
- Joint contractures
- Hypoglycemia/hyperglycemia
- Diabetic ketoacidosis
- Non-ketotic hyperosmolar state
Macrovascular:
- Ischemic heart disease
- Hypertension
- Stroke
- Peripheral vascular disease
Microvascular:
- Peripheral vascular disease
- Nephropathy and renal failure
- Retinopathy
- Neuropathy (autonomic and somatic)
Contraindications to Pancreas Transplantation
Absolute Contraindications
- Untreatable coronary disease
- Irreversible pulmonary and hepatic dysfunction
- Recent myocardial infarction or poor left ventricular function
- Active infection
- Malignancy within the last 3 years
- Positive HIV serology or hepatitis B
- Substance abuse or severe psychiatric illness
Relative Contraindications
- Age >55 years
- Recent retinal hemorrhage
- Symptomatic cerebrovascular or peripheral vascular disease
- BMI >30 kg/m²
- Smoking
- Severe aorto-iliac disease
Procedure
- The surgery typically lasts 5–7 hours.
- Bench preparation of the pancreas allograft takes up to 2 hours and includes removal of the spleen, mobilization of the portal vein, and restoration of arterial vascular anatomy using the donor iliac vessels to form a Y-shaped graft.
- An extended Pfannenstiel incision or, in the case of SPK, a midline incision from the pubis to 5 cm above the umbilicus is made. It is an intraperitoneal procedure with division of the musculature and mobilization of the right-sided iliac vessels.
- The portal vein is anastomosed to the external iliac vein, and the donor end of the common iliac artery is sutured to the recipient common iliac artery. In SPK, the kidney is placed in the left iliac fossa.
- The preferred method of duct drainage is enteric, with the duodenum anastomosed to the recipient’s ileum.
- Previously used bladder drainage caused complications like urethral strictures, cystitis, excessive bicarbonate losses, and metabolic acidosis in 40% of recipients but is still used for isolated pancreas transplants.
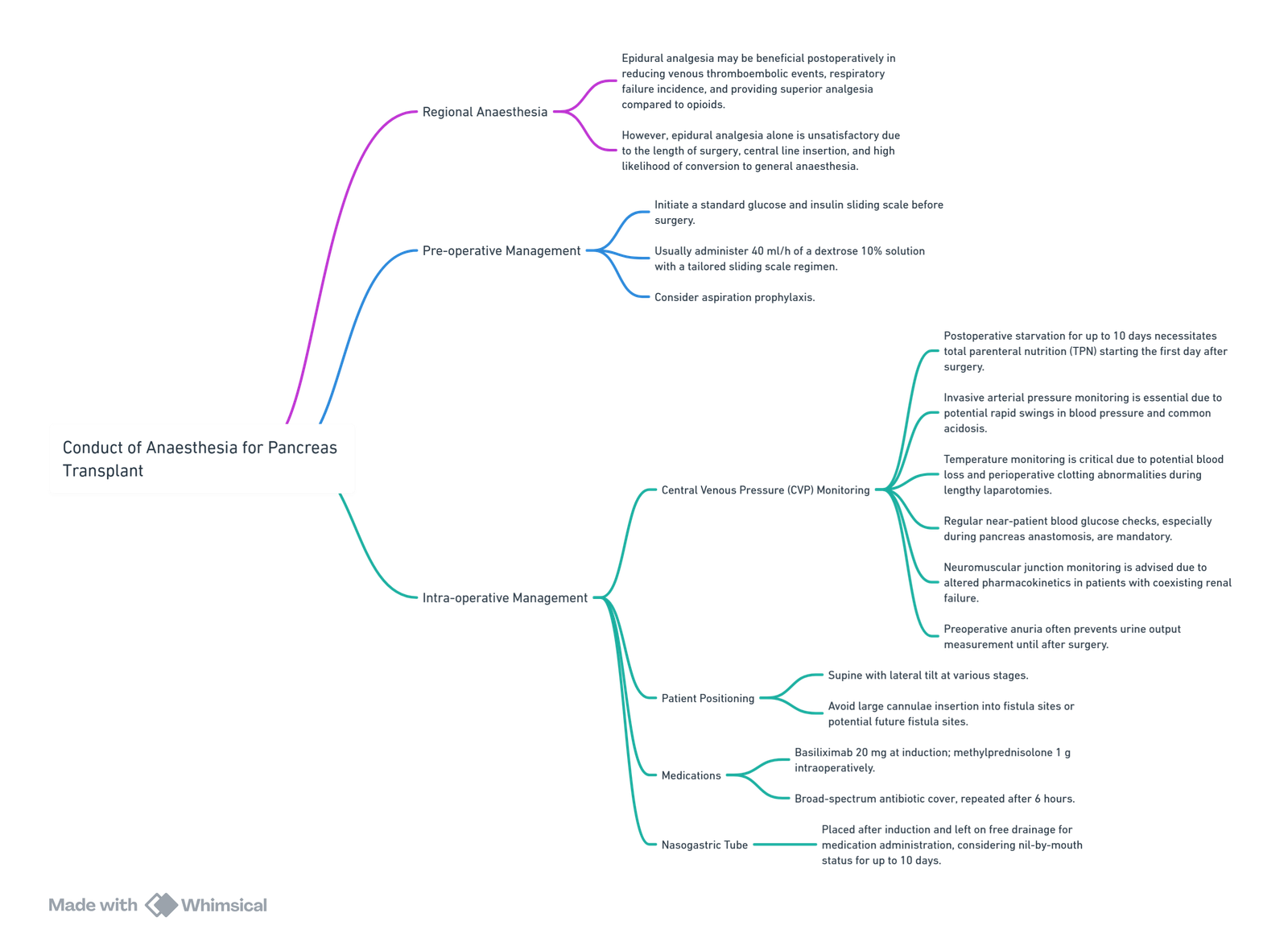
Conduct of Anaesthesia
View or edit this diagram in Whimsical.
Specific Complications of Pancreas Transplantation
- Graft thrombosis
- Pancreatitis
- Local and systemic infection
- Leaks from duodenal/ileal anastomosis
- Side effects of immunosuppressants
- Bladder problems (if bladder drained and bicarbonate losses)
- Systemic inflammatory response syndrome
Post-operative Management
- Total parenteral nutrition (TPN) and low molecular weight heparin (LMWH) for thromboprophylaxis are typically started on the first day after surgery.
Links
- Transplants and organ donation
- Liver resection
- Liver transplant
- Liver physiology and pathology
- ICU and liver disease
- Pancreatitis
- Endocrine and Metabolic
References:
- Powell, L., Hood, G., & Wyman, A. (2009). Gastrectomy for adenocarcinoma. Continuing Education in Anaesthesia Critical Care &Amp; Pain, 9(2), 65-69. https://doi.org/10.1093/bjaceaccp/mkp007
- Pichel, A. and Macnab, W. R. (2005). Anaesthesia for pancreas transplantation. Continuing Education in Anaesthesia Critical Care &Amp; Pain, 5(5), 149-152. https://doi.org/10.1093/bjaceaccp/mki040
- How I do it: Anaesthesia for Whipple Procedure Dr Sylvia Haijke. UCT refresher 2015
- PERIOPERATIVE MANAGEMENT OF PATIENTS UNDERGOING PANCREATICODUODENECTOMY Dr Howard Radford. Wits refresher 2013
- Southern African Journal of Anaesthesia and Analgesia 2017; 23(2)(Supplement 1). Carcinoid syndrome M A Eagar
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org/
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
Summaries:
Gastrectomy
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “6e227da9-6282-4737-aafd-45528bfa7a13”



