- Summary
- Introduction
- Antepartum Haemorrhage
- Post Partum Haemorrhage
{}
Summary
Introduction
- Haemorrhage—antepartum, intrapartum and postpartum—remains the leading cause of maternal mortality worldwide, accounting for approximately 27% of maternal deaths, with proportions exceeding 30% in sub‐Saharan Africa and South Asia.
- Global incidence of postpartum haemorrhage (blood loss > 500 mL after vaginal delivery or > 1 000 mL after caesarean delivery) is 10.8%, with severe cases (> 1 000 mL) in 2.8% of deliveries.
General Anaesthetic Considerations for Placental Pathology
Pre‐operative Assessment and Preparation
- Full blood count, coagulation profile, group and save, cross‐match (minimum 4 units).
- Ensure availability of cell salvage, rapid infusers and blood‐warming devices.
- Optimise antenatal anaemia with intravenous iron or erythropoietin where indicated.
Pharmacological Haemostatic Strategies
- Tranexamic acid (TXA): 1 g IV infusion over 10 minutes within 3 hours of bleeding onset; repeat 1 g if bleeding persists after 30 minutes (WOMAN trial).
Choice of Anaesthetic Technique
- Neuraxial anaesthesia (spinal or combined spinal–epidural) is preferred for elective lower-segment caesarean section in placenta previa when coagulation parameters are normal:
- Associated with ~20% lower estimated blood loss and ~30% fewer transfusions compared with general anaesthesia.
- Conversion to general anaesthesia occurs in 10–20% of cases; counsel patients accordingly.
- General anaesthesia indicated for haemodynamic instability, ongoing massive haemorrhage or coagulopathy; rapid-sequence induction recommended
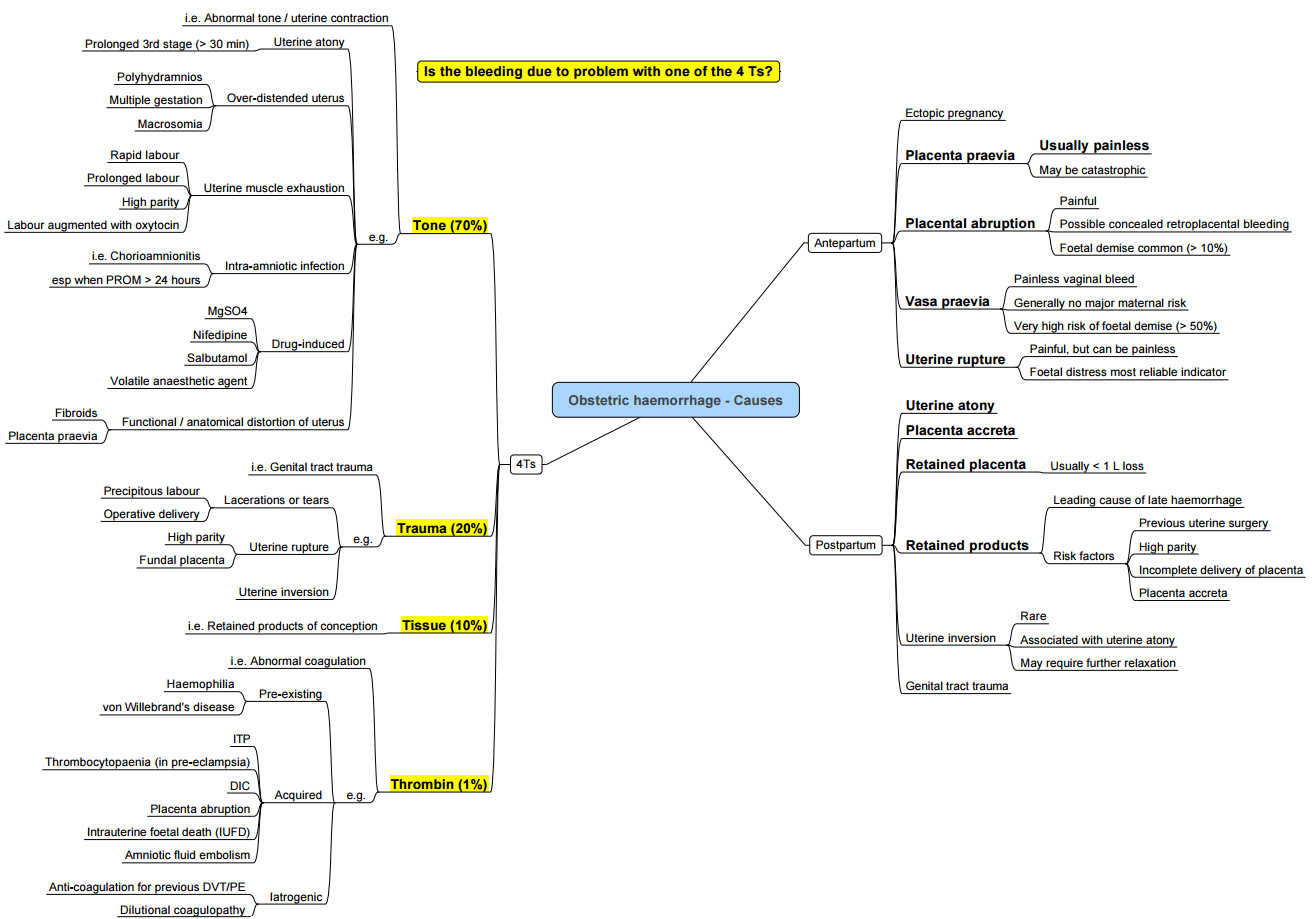
Antepartum Haemorrhage
- Bleeding after 20 weeks’ gestation and prior to delivery complicates ≈ 3–5% of pregnancies. Main causes:
- Placenta previa
- Placenta accreta spectrum
- Placental abruption
- Vasa praevia
- Uterine rupture
Placenta Previa
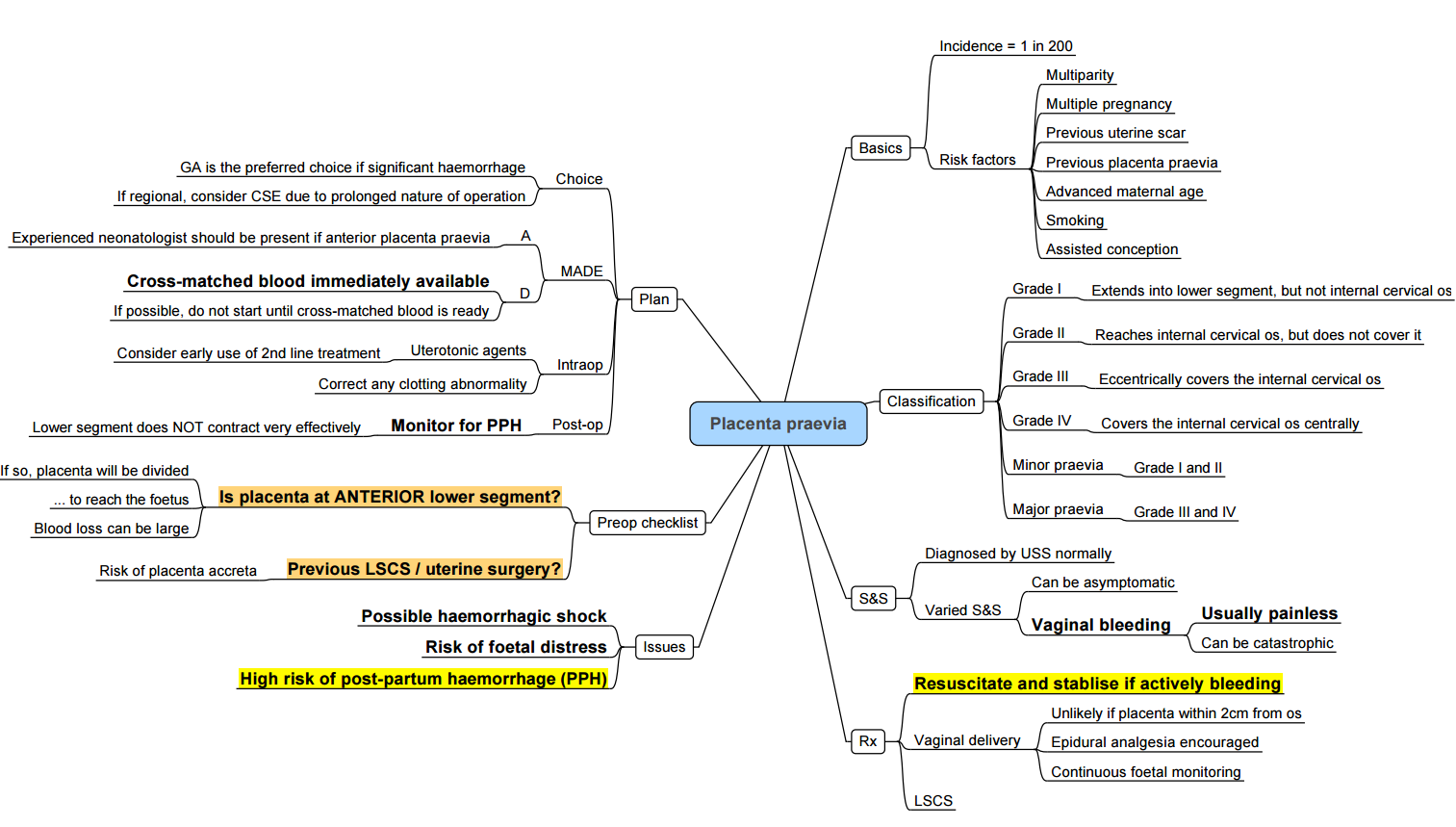
Definitions and Classification
- Placenta previa: placental tissue reaches or covers the internal cervical os.
- Types:
- Major (complete coverage)
- Partial
- Marginal (within 2 cm of os)
- Low-lying (2–5 cm from os in the third trimester)
Risk Factors
- Previous caesarean section
- Advanced maternal age
- Multiparity
- Multiple gestation
- Smoking
Diagnosis and Monitoring
- Transabdominal/transvaginal ultrasound at 18–20 weeks; repeat at 32 weeks to assess placental migration.
- Digital vaginal examination contraindicated until placenta previa excluded
Management
- Elective caesarean at 37 weeks in a multidisciplinary centre.
- Neuraxial anaesthesia if stable (see Section 1.3).
- Prepare for massive transfusion, cell salvage and invasive monitoring.
Placenta Accreta Spectrum
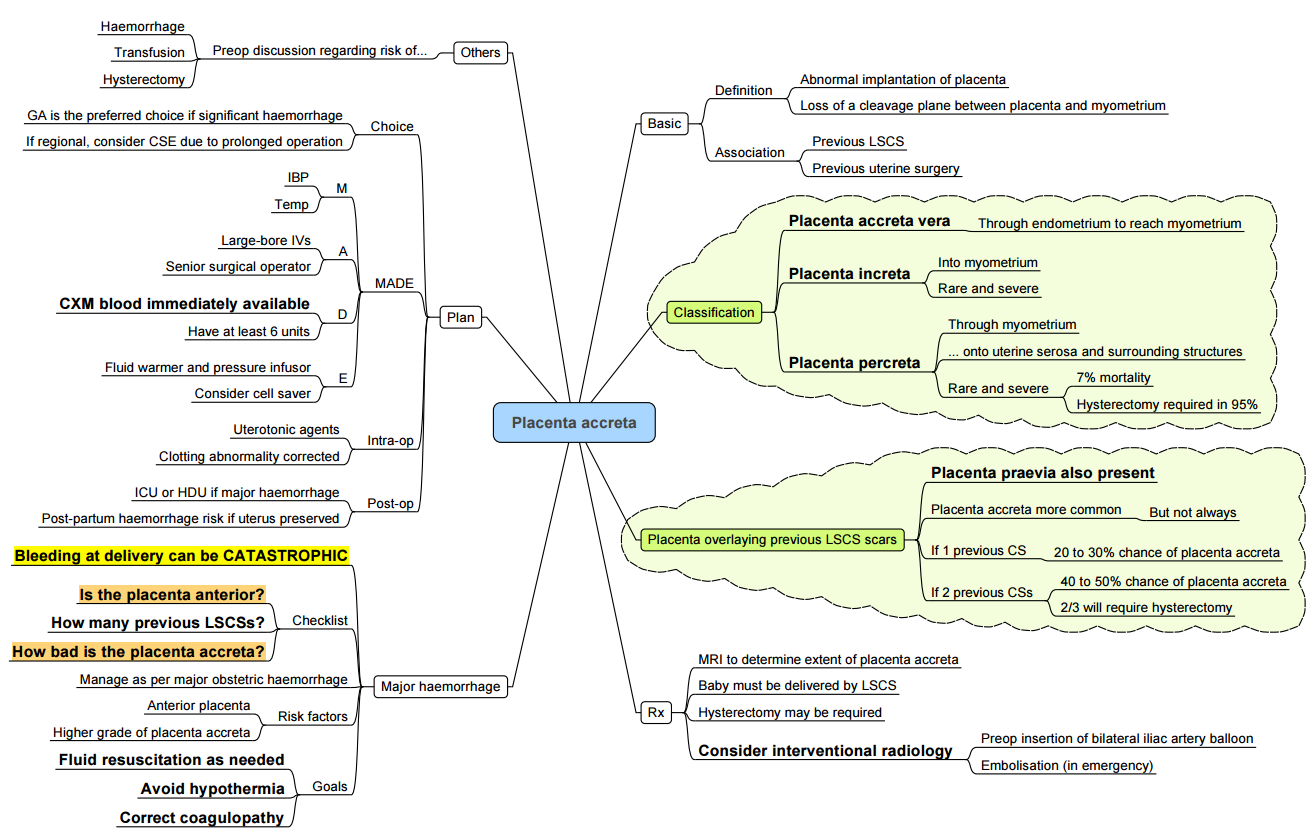
Incidence and Risk Factors
- Incidence: ~0.4–0.9% of pregnancies; increasing with caesarean rates.
- Risk factors: prior uterine surgery (caesarean, myomectomy), multiparity, placenta previa.
Classification
- Accreta: villi adhere to myometrium.
- Increta: invasion into myometrium.
- Percreta: penetration through uterine serosa ± adjacent organs.
Anaesthetic Considerations
- Planned delivery at 34–36 weeks in a centre of excellence with interventional radiology on standby.
- Neuraxial anaesthesia may be feasible if minimal anticipated blood loss.
- General anaesthesia with invasive arterial and central venous monitoring when massive haemorrhage is anticipated
- Adjuncts (balloon occlusion, cell salvage)
Placental Abruption
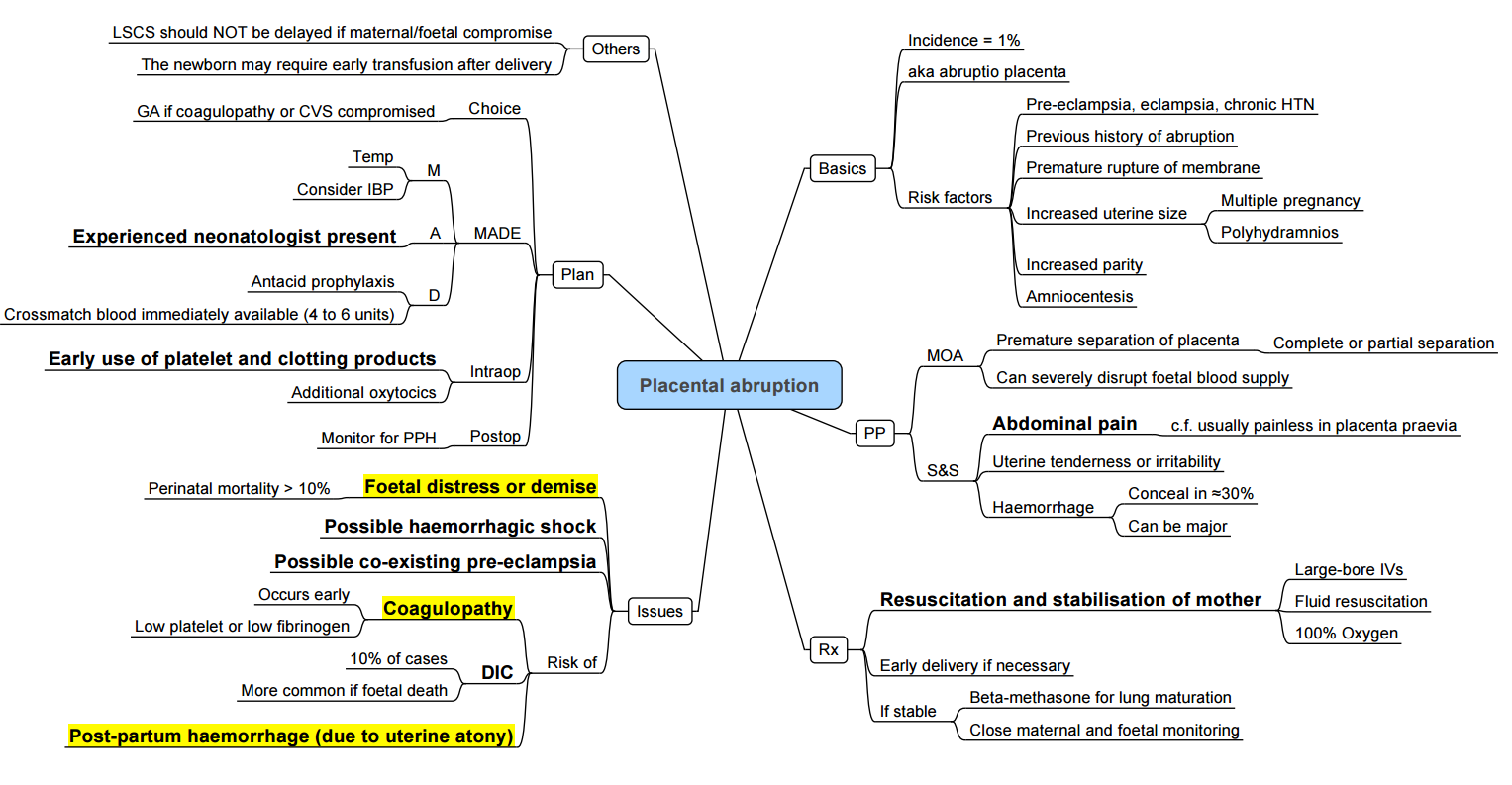
Incidence and Risk Factors
- Occurs in 0.6–1.2% of pregnancies.
- Risk factors: hypertension, trauma, pre-eclampsia, smoking, cocaine use, multiparity, short umbilical cord.
Clinical Presentation and Severity
- Vaginal bleeding ± uterine tenderness; may be concealed.
- Graded I–III (mild to severe) by volume of bleeding, uterine tone, maternal/fetal compromise
Management
- Immediate haemodynamic stabilisation and coagulation support.
- Emergency caesarean under general anaesthesia with rapid-sequence induction if maternal or fetal distress.
- Utilise massive transfusion protocols and point-of-care coagulation testing.
Uterine Rupture
Risk Factors and Presentation
- Risk factors: scarred uterus (prior caesarean/myomectomy), obstructed labour, oxytocin-induced hypertonus.
- Presentation: sudden abdominal pain, loss of fetal station, bleeding, shock.
Management
- Emergency laparotomy and delivery under general anaesthesia.
- Be prepared for hysterectomy and massive blood replacement.
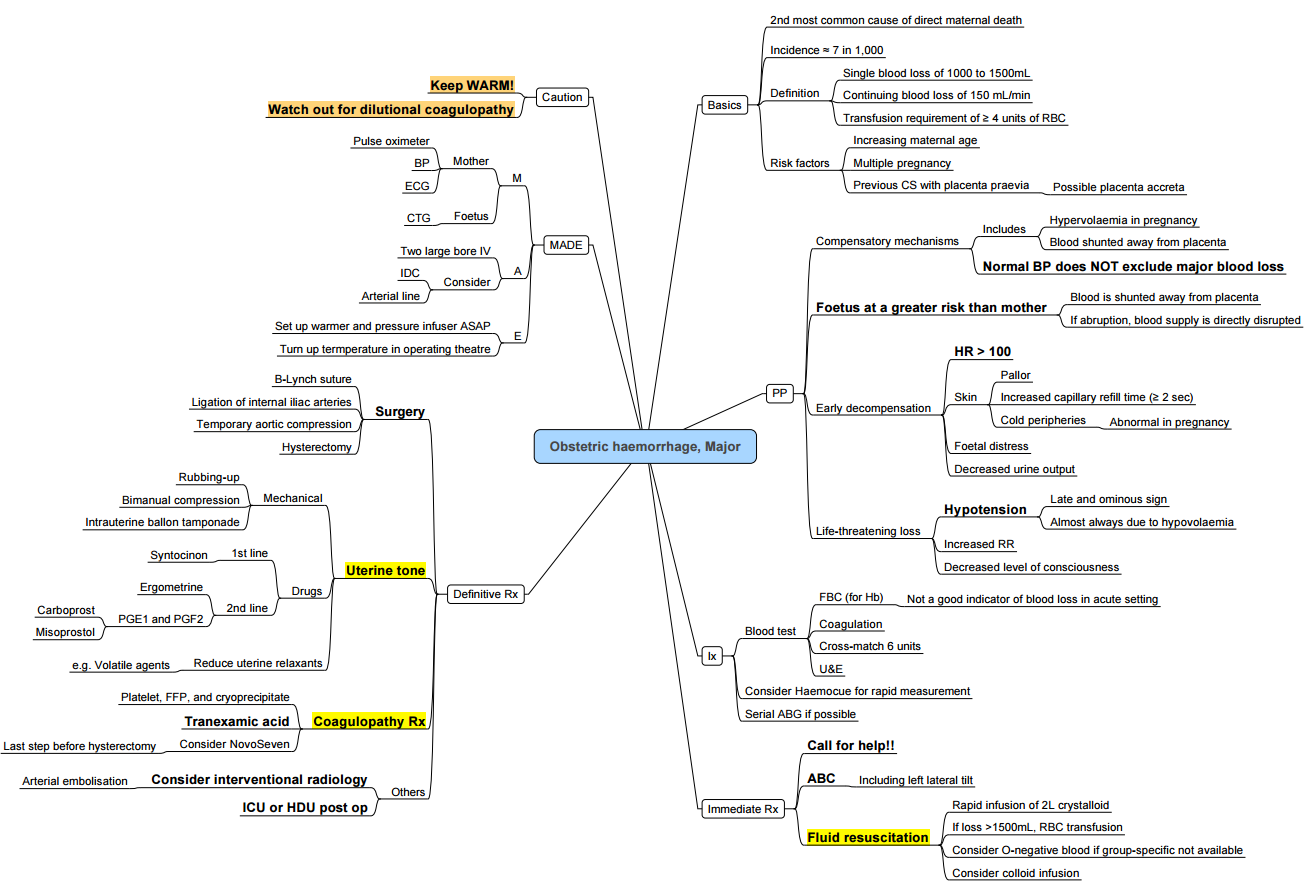
Post Partum Haemorrhage
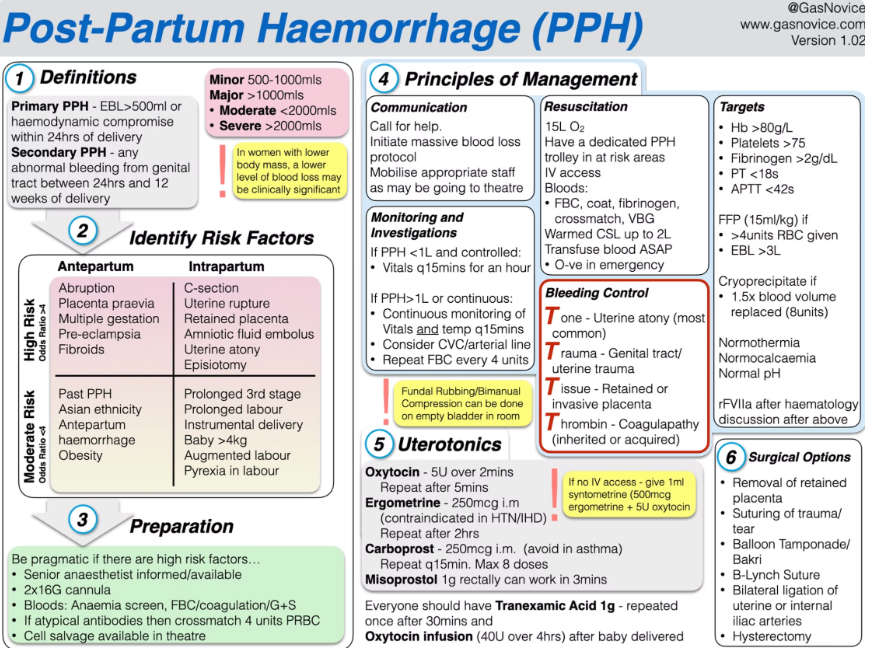
Definition
- Postpartum haemorrhage (PPH) is defined by the American College of Obstetricians and Gynecologists as cumulative blood loss ≥ 1000 mL or any blood loss accompanied by signs or symptoms of hypovolaemia within 24 hours of delivery, irrespective of mode of birth
- Severe PPH: blood loss > 2000 mL and/or transfusion of ≥ 4 packed red blood cells (PRBCs).
- Timing:
- Primary (early): within 24 hours of delivery
- Secondary (late): > 24 hours and up to 12 weeks postpartum
Pathogenesis (the “Four T’s”)
- Tissue
- Retained placental fragments prevent adequate myometrial contraction and vessel compression.
- Risk factors: manual removal of placenta, placenta accreta spectrum, uterine atony.
- Thrombin (coagulopathy)
- Consumptive coagulopathy (e.g. disseminated intravascular coagulation), pre‐existing bleeding disorders.
- Obstetric triggers: placental abruption, amniotic fluid embolism, pre‐eclampsia.
- Tone (uterine atony)
- Most common cause (≈ 70% of PPH).
- Predisposing factors: over-distension (multiple gestation, polyhydramnios, macrosomia), prolonged or augmented labour (oxytocin), general anaesthesia.
- Trauma
- Genital tract lacerations, uterine rupture, instrumental delivery, caesarean section.
- Additional risk factors include maternal age > 34 years, multiparity, fibroids, smoking, chorioamnionitis, and hypertensive disorders of pregnancy.
Clinical Recognition
- Visual estimation of blood loss underestimates actual loss by up to 50%.
- Rule of 30 (≈ 30% of maternal blood volume lost):
- Haemoglobin ↓ 30%
- Urine output < 30 mL/h
- Heart rate ↑ 30 bpm
- Systolic blood pressure ↓ 30 mmHg
- Respiratory rate > 30 breaths/min
- Shock index (heart rate ÷ systolic blood pressure)
- Normal ≤ 0.9; > 1.7 predicts need for ≥ 4 units PRBCs, hysterectomy, ICU admission, mortality
Management Algorithm
Step 1: Recognition and Initial Resuscitation (≤ 30 min)
- Call for help: obstetric, anaesthetic and haematology teams.
- Monitoring: blood pressure, heart rate, oxygen saturation, urine output, shock index.
- Establish two large-bore IV lines; send for full blood count, coagulation screen, group and cross-match.
- Send fixed-ratio red cell and plasma packs via massive transfusion protocol (MTP).
- Uterine massage and manual removal of retained tissue if indicated.
Step 2: Uterotonic Agents
- Administer sequentially if tone remains inadequate:
- Oxytocin: 10–30 IU in 1 L crystalloid infusion (slow infusion; bolus avoided).
- Ergometrine (methylergometrine) 0.2 mg IM (contraindicated if hypertension).
- Carboprost (15-methyl prostaglandin F₂α) 250 µg IM every 15–30 min up to 2 mg total (contraindicated in asthma).
- Misoprostol 800 µg rectally or sublingually.
Step 3: Haemostatic Support and Monitoring (next 30 min)
- Point-of-care coagulation testing (TEG/ROTEM) to guide component therapy:
- Maintain fibrinogen ≥ 4 g/L (normal in term pregnancy 4.5–6 g/L).
- Platelets ≥ 75 × 10⁹/L; PT/INR < 1.5 × normal; aPTT < 1.5 × normal.
- Tranexamic acid 1 g IV over 10 min; may repeat once.
- Fibrinogen concentrate or cryoprecipitate to restore fibrinogen.
- Consider recombinant factor VIIa only after correction of acidosis, hypothermia, thrombocytopenia and hypofibrinogenaemia.
Step 4: Mechanical and Surgical Interventions
- Mechanical tamponade: intrauterine balloon (e.g. Bakri).
- Compression sutures: B-Lynch or modified sutures.
- Arterial ligation: uterine or internal iliac arteries.
- Hysterectomy for life-threatening haemorrhage unresponsive to conservative measures.
- Consider interventional radiology (embolisation) if stable and available.
Massive Transfusion Protocol
- Initial pack: 6 units O RhD-negative red cells, 4 units AB plasma, 1 apheresis platelet unit.
- Ratio-based vs goal-directed: current evidence favours point-of-care–guided therapy to minimise volume overload and TRALI.
Blood Conservation Strategies
- Antepartum: screen and treat iron-deficiency anaemia (target Hb ≥ 11 g/dL).
- Postpartum: consider IV iron before transfusion if stable.
- Cell salvage: safe in high-risk caesarean sections with leukocyte filtration and appropriate Rho(D) immunoglobulin.
Cyklokapron (Tranexamic Acid) in Obstetric Cesarean Section – Evidence Summary
| Category | Details / Evidence |
|---|---|
| Primary Use | Prophylactic TXA (Cyklokapron) at 1 g IV before skin incision, alongside uterotonic therapy in cesarean delivery. |
| Effect on Blood Loss | Meta-analysis of ~50 RCTs showed reduced risk of blood loss > 1000 mL, lower mean blood loss, reduced transfusion needs, and less drop in hemoglobin, with greater benefit in high‑risk patients. |
| Timing of Administration | Benefit seen when TXA given before skin incision; no clear benefit if administered after cord clamping. |
| Maternal Mortality / Clinical Endpoints | Large trials (e.g. TRAAP‑2, NIH-MFM Units Network) confirmed reduced blood loss but no significant reduction in mortality or composite need-for-transfusion endpoints. |
| Safety & Thrombotic Risk | No significant increase in thromboembolic events overall; incidence remains low and statistically nonsignificant. Minor GI side effects may be modestly increased. |
| Evidence Quality | Moderate quality for high‑risk populations; overall evidence for low-risk populations rated low to very low. |
| Guideline Status | WHO and obstetric panels continue to support IV TXA for treatment of PPH. Prophylactic use in Cesarean section remains conditionally recommended, especially in high‑risk scenarios. |
| Outstanding Research | Ongoing trials (e.g. TRAAP‑VIA, I’M WOMAN) focusing on placenta previa, anaemic populations, and alternative TXA routes; results expected by late 2025. |
Practice Conclusion
- Use of prophylactic IV TXA before incision in Cesarean delivery reduces hemorrhage and transfusion needs, with greater benefit in high-risk patients.
- The effect on clinical mortality or composite transfusion endpoints remains unproven.
- Thromboembolic risk is not significantly elevated under current dosing and use patterns.
- Optimal dosing, high-risk subgroup targeting, and administration timing continue to be refined in ongoing trials.
Links
- Polytrauma and haemorrhagic shock
- Point of Care Coagulation testing
- Clotting cascade
- Resus end targets in shock
- Obstetric emergencies
- Blood conservation in cardiac surgery
- Blood transfusions and conservation strategies
- Hypertension in pregnancy
References:
- Velde, M. V. d. and Varon, A. J. (2015). Obstetric hemorrhage. Current Opinion in Anaesthesiology, 28(2), 186-190. https://doi.org/10.1097/aco.0000000000000168
- Van de Velde, Marca; Diez, Christianb; Varon, Albert J.b. Obstetric hemorrhage. Current Opinion in Anaesthesiology 28(2):p 186-190, April 2015. | DOI: 10.1097/ACO.0000000000000168
- Butwick, Alexander J.a; Goodnough, Lawrence T.b,c. Transfusion and coagulation management in major obstetric hemorrhage. Current Opinion in Anaesthesiology 28(3):p 275-284, June 2015. | DOI: 10.1097/ACO.0000000000000180
- The Calgary Guide to Understanding Disease. (2024). Retrieved June 5, 2024, from https://calgaryguide.ucalgary.ca/
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org/
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
- PPH Image: Novice Anaesthesia. (2021). Infographics. Retrieved April 24, 2025, from https://www.gasnovice.com/infographics
- Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum haemorrhage. Green-top Guideline No. 52. London: RCOG; 2017.
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: WHO; 2019.
- Roberts I, Shakur H, Afenyi-Annan A, et al. The WOMAN Trial: tranexamic acid for the treatment of postpartum haemorrhage. Lancet. 2017;389(10084):2105–16.
- Collins PW, et al. Early fibrinogen concentrate replacement in postpartum haemorrhage: a randomised controlled trial. Br J Anaesth. 2016;116(6):804–12.
- Sentilhes L, Kayem G, Perrotin F, et al. Conservative management of severe postpartum haemorrhage using intrauterine balloon tamponade. Obstet Gynecol. 2016;127(4):613–19.
Summaries
Obs_Haemorrhage
—
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “dc9a2130-ce3c-4e2a-b8ef-5ab01bac8872”



