- Aortic Aneurism
- Conduct of Anaesthesia for an Aortic Aneurism Repair
- Pre Operative Management
- Initial Management of Patients with Suspected Aortic Dissection
- Risk Stratification
- Preoperative Assessment
- Preparation
- Intraoperative Management
- Postoperative Management
- Complications
- Management Summaries
- Pre Operative Management
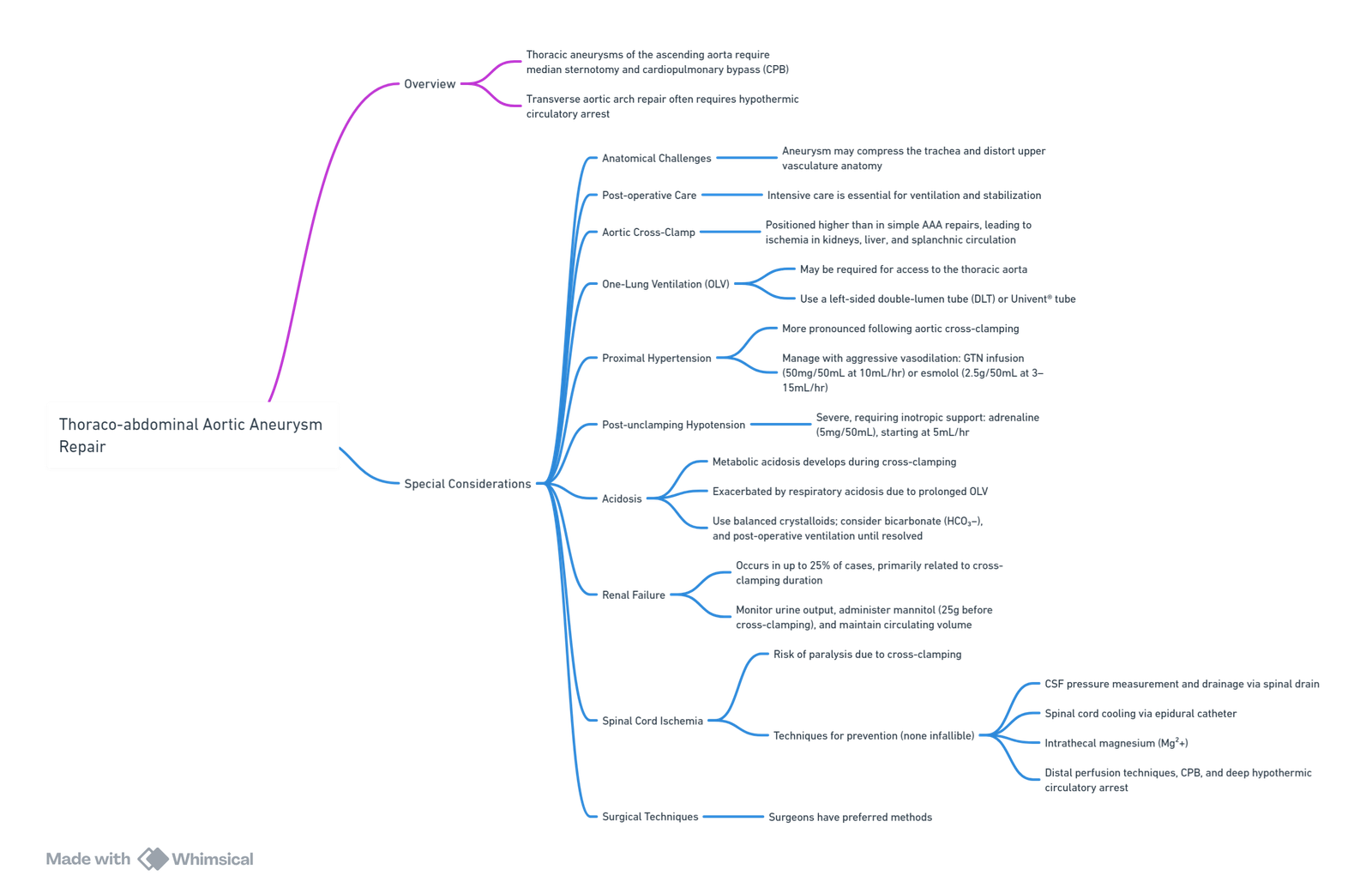
- Thoraco-abdominal Aortic Aneurysm Repair
- Links
- Past Exam Questions
{}
Aortic Aneurism
Definition and Classification
Aortic Aneurysm
- True aneurysm: Irreversible dilation of all three layers of the vessel wall, exceeding 50% of the normal diameter.
- Pseudoaneurysm: Rupture through vessel layers, held by surrounding tissue
- Normal aortic diameter ~2 cm; aneurysm defined as ≥3 cm.
Thoracoabdominal Aortic Aneurysm (TAAA)
- Involves visceral arteries (coeliac, SMA, renal).
- Crawford Classification:
- Extent I: Left subclavian to below diaphragm.
- Extent II: Left subclavian to aortic bifurcation.
- Extent III: Lower descending thoracic aorta to aortic bifurcation.
- Extent IV: Abdominal aorta only.
Aortic Dissection
- Disruption of intima, blood enters media creating a false lumen.
- DeBakey Classification:
- Type I: Ascending, arch, descending.
- Type II: Ascending only.
- Type III: Descending only.
- Stanford Classification
- Type A: Any ascending aorta involvement.
- Type B: Distal to left subclavian.
Epidemiology and Natural History
- Untreated TAAA >6 cm: 14.1% annual risk of rupture or death.
- Conservative 5-year survival: 10–20%.
- Indications for surgery:
- Rupture, dissection.
- Symptomatic or rapid expansion (>1 cm/year).
- Diameter >6.5 cm (or >6.0 cm in connective tissue disorders).
Pathophysiology
Aneurysm Development
- Connective tissue degradation, biomechanical stress, inflammation.
- Risk factors: hypertension, atherosclerosis, genetic syndromes (Marfan, Ehlers-Danlos, Loeys-Dietz, Turner).
Aortic Wall Changes
- Loss of smooth muscle, elastin degradation, thinning of media.
- Infrafrenal aorta: fewer lamellar units, poor vasa vasorum, more prone to dilation.
Aortic Dissection
- Triggered by hypertension, trauma, or iatrogenic causes.
- Intimal tear causes propagation into media.
- Potential complications:
- Aortic rupture.
- Tamponade.
- Malperfusion syndromes.
- Aortic regurgitation.
- Neurological sequelae (e.g. Horner’s, hoarseness).
Clinical Presentation
- Asymptomatic until rupture.
- Symptoms: pain (back, chest), compressive features (stridor, hoarseness, dysphagia).
- Rupture syndromes:
- Posterior: retroperitoneal tamponade.
- Anterior: intraperitoneal bleeding, rapid death.
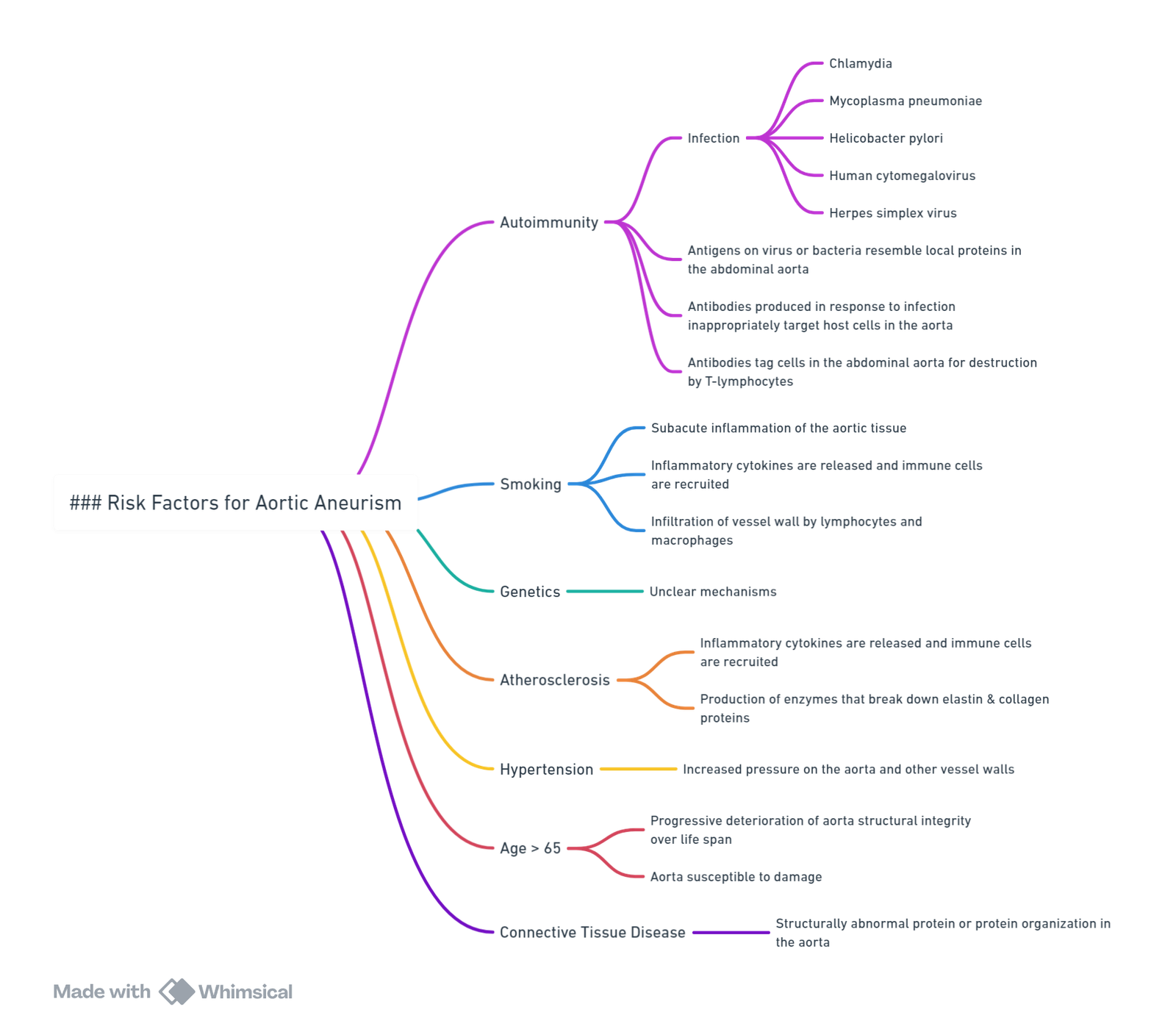
Risk Factors
Ascending Vs Descending Disease
| Disease | Approach | Position on Table | Use of Bypass | Heparinization | Hypothermia | Active Neuroprotection |
|---|---|---|---|---|---|---|
| Ascending and Arch Disease | Sternotomy | Supine | Full cardiopulmonary bypass | Yes | Yes | Variable |
| Descending Disease | Left thoracotomy | Lateral | Usually none, may be shunts or partial bypass | Variable | Usually no active measures | Variable |
Conduct of Anaesthesia for an Aortic Aneurism Repair
Pre Operative Management
Initial Management of Patients with Suspected Aortic Dissection
- Airway and Breathing
- Administer supplemental oxygen if hypoxia
- Secure the airway if there is respiratory compromise or altered consciousness.
- Circulation and Monitoring
- Attach continuous ECG, BP, and SpO₂ monitors.
- Insert two large-bore i.v. lines.
- Take urgent bloods: crossmatch, troponin, D-dimer, CK, FBC, U&E, LDH, myoglobin, coagulation profile.
- Pain Control
- Administer i.v. opioids (e.g. morphine) to reduce pain and sympathetic drive, which helps control BP and HR.
- Haemodynamic Management
- Target HR <60 bpm and SBP 100–120 mmHg to reduce shear stress on the aortic wall.
- In acute dissection consider SBP 80-90 mmHg (Permissive hypotension)
- First-line: Beta-blockers
- Esmolol (short-acting) or labetalol (combined alpha and beta blocker).
- If beta-blockers are contraindicated:
- Add calcium channel blockers (e.g. diltiazem or verapamil).
- Vasodilators (e.g. sodium nitroprusside) may be added only after beta-blockade is established to avoid reflex tachycardia.
- Avoid excessive fluid loading unless hypotensive, as it may worsen dissection or precipitate rupture.
- Target HR <60 bpm and SBP 100–120 mmHg to reduce shear stress on the aortic wall.
- Electrocardiogram (ECG)
- Rule out myocardial infarction (especially in proximal dissections).
- Imaging
- Obtain CT angiography (if stable), or transoesophageal echocardiography (TOE) if unstable or CT unavailable.
- Definitive Management
- Type A (ascending aorta): Emergency surgery.
- Type B (descending aorta): Medical management unless complications arise (e.g. rupture, malperfusion, aneurysm expansion).
- Transfer
- Urgent transfer to cardiothoracic or vascular surgical center if not already on site. Involve ICU and cardiothoracic team early.
Risk Stratification
Glasgow Aneurysm Score (GAS)
- Age of patient (points = no. of years): 1 point per year
- Shock: 17 points
- Myocardial disease: 7 points
- Includes previous myocardial infarction and/or ongoing angina.
- Cerebrovascular disease: 10 points
- Includes all grades of stroke, including transient ischaemic attack.
- Renal disease: 14 points
- Defined as serum urea > 20 mmol/L and/or creatinine > 150 mmol/L.
- Total Score Calculation:
- Age in years is added to the other variables to produce a total score.
- The best cut-off value for GAS in emergency repair is 84, indicating a mortality of 65%.
- Predictive Utility:
- GAS is useful in predicting postoperative mortality in both elective and emergency AAA repair.
- Postoperative mortality:
- Elective repair: 1.4% in patients with a GAS < 78.8; 8.7% in those with a GAS ≥ 78.8.
- Emergency repair: 28% with a GAS < 84; 65% with a GAS ≥ 84.
Hardman Index
- Age > 76: 1 point
- Serum creatinine > 190 µmol/L: 1 point
- Haemoglobin < 9 g/dL: 1 point
- Myocardial ischaemia on ECG: 1 point
- A history of loss of consciousness after arrival to hospital: 1 point
- Total Score Calculation:
- Each preoperative variable present is assigned 1 point, with a possible score ranging from 0 to 5.
- A total score of ≥ 2 is consistent with a mortality rate of > 80%.
- Predictive Utility:
- Recent studies have predicted a mortality of 80% with a Hardman index score of ≥ 2.
Preoperative Assessment
- Multisystem evaluation; screen for CAD, pulmonary disease.
- Pulmonary function, coronary angiography/CT as indicated.
- Baseline neurology.
- TOE/TTE for cardiac and aortic assessment.
- Co-morbidities
- Coronary artery disease (CAD)/ischemic heart disease (IHD)
- Smoking
- Chronic obstructive pulmonary disease (COPD)
- Diabetes mellitus (DM)
- Hypertension (HT)
- Haemodynamic
- SBP 100-120, Pulse <60
- In acute dissection consider SBP 80-90 mmHg (Permissive hypotension)
- Beta blockers then vasodilators
- SBP 100-120, Pulse <60
Preparation
Personnel
- Assistance: 2 senior anaesthetists
Equipment (Theatre Preparation)
- Bloods and Fluids:
- At least 10 units of red blood cells, platelets, fresh frozen plasma, and cryoprecipitate.
- Avoid clear fluids.
- Do not transfuse preoperatively unless absolutely necessary.
- Use cell salvage as it reduces the use of allogeneic blood in aortic surgery.
- Warmers:
- Hot-air and IVI warmers are essential. Monitor intraoperative temperature.
- IV Access:
- Two 14G or greater IV accesses.
- Cell Saver
- Syringe Drivers
- At least two for inotropes, vasodilators, and epidural.
- Rapid Infusion System
- A-line and CVC
- Triple-lumen CVP after induction.
- Ultrasound
- TEG
- Lumbar Drain
- Indications include increased risk of spinal cord injury (SCI), Type 1 Crawford thoracoabdominal aortic aneurysm (TAAA), prior distal aortic repair.
- Medications:
- Vasoconstrictors (ephedrine and metaraminol), vasodilators (GTN), and β-blockers (labetalol).
- Analgesia:
- Consider preoperative epidural catheter in patients with a contained leak if coagulation results are satisfactory and the patient is hemodynamically stable.
- Thoracic epidural (T6–T11) pre-induction.
- PAFC Introducer:
- For complex cases, allows rapid fluid administration and PA catheter insertion if necessary.
- Continuous Cardiac Output Monitoring:
- Useful during the cross-clamp period, especially for patients with impaired cardiac function.
- Options include PA catheter, LiDCO™, PiCCO™, and esophageal Doppler (not accurate during aortic cross-clamping).
Intraoperative Management
- Airway
- Double-lumen tube (DLT), consider right-sided if left bronchial distortion on CXR.
- Position
- Right lateral (left side up).
- Temperature
- Permissive cooling to reduce ischemic risk.
- Fluids
- Adequate hydration to reduce renal impairment risk post-op; use rapid infusion system.
- IV Access
- Wide-bore peripheral venous cannulae and central venous pressure (CVP) line.
- Blood Management
- Cell salvage, rapid infusion system, cross-matched blood in OR.
Monitors
- Transesophageal echocardiogram (TOE): LV function, filling, assess dissection, confirm CVP position, repair quality.
- Arterial line (A-line): Right radial (proximal aneurysm pressure), right femoral (distal perfusion).
- Point-of-care monitoring: ABG, TEG, BSL, HB, Electrolytes.
- Cerebral oximetry: If cross-clamp close to origins of the left common carotid.
- Intracerebral oxygen saturation (rSO2) is calculated and should be maintained within 25% of baseline
- Depth of anaesthesia monitoring.
- Neuromuscular transmission monitor.
- CSF drain.
- +/- Pulmonary artery catheter.
Induction
- Only induce when setup and hemodynamic control are assured.
- Cardiovascular collapse risk due to:
- Cardiodepressant effects of intravenous and inhalational agents.
- Abdominal muscle relaxation reducing tamponade effect.
- Intermittent positive pressure ventilation reducing venous return.
- Reduction in sympathetic tone.
- Modified RSI with careful induction, monitoring invasive arterial BP.
- Use moderate/high-dose opioid, e.g., remifentanil (0.1–0.2 µg/kg/min) or high-dose fentanyl (5–10 µg/kg).
- Treat hypotension with fluids first, then cautious vasoconstriction (metaraminol 0.25–0.5 mg).
- No difference in myocardial outcome between sevoflurane-based anaesthesia and TIVA.
- Hypothermia likely unless actively managed.
- Avoid warming blankets on lower limbs during aortic cross-clamp to prevent worsening ischemia.
- Insert urinary catheter for hourly urine output measurements.
Maintenance
- General anesthesia (GA) alone or GA with epidural: Pros = good analgesia, less respiratory depression; Cons = masks motor block secondary to spinal ischemia.
- Balanced technique using volatile agents/opioids and neuromuscular blockade.
- Avoid N2O.
- Acid-Base Management: Ischemia causes acidosis; may need bicarbonate.
- Glucose Control: Insulin infusion.
- Electrolyte Management: Monitor for hypocalcemia (citrate accumulation) and hypomagnesemia.
- Hemodynamic Control:
- Monitor proximal MAP (80–90 mmHg), distal (60–70 mmHg).
- Maintain high upper body MAP (80-90 mmHg) for increased collateral flow to anterior spinal arteries.
- Aim for 60 mmHg distal to cross-clamp.
- Consider partial CPB or shunts depending on surgical site.
- Have adrenaline, noradrenaline, dobutamine, esmolol, and GTN ready.
- Monitor proximal MAP (80–90 mmHg), distal (60–70 mmHg).
- Renal and Spinal Protection:
- Cold perfusion of kidneys during ischemia time & CPB/shunt.
- MEPs & SSEPs, CSF drain, permissive hypothermia, selective reimplantation of intercostal vessels.
- Maintain MAP as above, use sequential cross-clamping techniques.
Spinal Cord Considerations
Background
- Paraplegia post-surgery occurs in 4–16% of cases overall, up to 50% in Extent II aneurysms.
- Mechanism: Aortic cross-clamping → ↓ arterial blood flow + ↑ CVP → ↓ spinal cord perfusion.
- Pathophysiology
- Aortic surgery disrupts arterial collaterals → ischaemia → reperfusion injury.
- Ischaemia → spinal oedema, hyperaemia, inflammation → ↑ CSFP → ↓ SCPP.
Risk Factors for Spinal Cord Ischaemia
- Aneurysm extent
- Long aortic cross-clamp time
- Emergency surgery
- Prior distal aortic surgery
- Severe peripheral vascular disease
- Perioperative hypotension
- Advanced age
- Diabetes mellitus
Strategies to Prevent Spinal Cord Ischaemia
- Aortic Clamping Strategy
- Sequential aortic clamping
- Reimplantation of intercostal/lumbar segmental arteries
- CSF Drainage
- SCPP = MAP–CSFP
- Target SCPP: ≥70 mm Hg
- Maintain:
- MAP ≥ 80 mm Hg
- CSFP < 15 mm Hg
- CSF drained at ≤20 mL/h, typically for up to 72 h post-op.
- Maintain:
- Technique
- Intrathecal catheter at L3–4 or L4–5
- Transduced CSFP monitoring
- Complications
- Spinal headache
- Neuraxial haemorrhage or haematoma
- Meningitis
- Intracranial hypotension
- Catheter fracture
- Interventions
- If CSFP >15 mm Hg: further ↑ MAP
- In suspected ischaemia: ↑ SCPP and MAP in 5 mm Hg increments
- Maintain MAP > 80 mmHg, Hb > 100 g/L.
- Consider neuroprotective agents: thiopental, methylprednisolone, lidocaine
- Neurophysiological Monitoring
- Motor-evoked potentials (MEPs):
- Monitor anterior spinal cord (motor pathways)
- Affected by NMBDs and volatile agents (TIVA preferred)
- Somatosensory-evoked potentials (SSEPs):
- Monitor posterior sensory columns
- Not affected by NMBDs or volatile agents
- Motor-evoked potentials (MEPs):
- Interpretation
- Greater than 50% ↓ in MEP amplitude:
- Reimplant intercostal arteries
- Optimise perfusion (MAP >80 mm Hg; distal aortic pressure >60 mm Hg)
- CSF drainage up to 20 mL/h
- Maintain Hb ≥ 100 g/L
- Greater than 50% ↓ in MEP amplitude:
- Timing
- Critical window: irreversible cell death occurs within 3–5 min of ischaemia onset
- Surgical action required promptly based on neurophysiological changes
- ICU management
- Monitoring
- Continue MEPs, SSEPs, CSFP, and ICP for up to 72 h
- Assess lower limb power regularly
- Early detection critical: delayed paraplegia has better prognosis
- COPS Protocol
- CSF Drain: Check patency, maintain CSFP <5 mm Hg
- Oxygen Delivery:
- Maintain SpO₂ >95%
- Supplemental O₂ or mechanical ventilation as required
- Patient Status:
- MAP >90 mm Hg
- SCPP >80 mm Hg
- Haemoglobin ≥120 g/L
- Cardiac Index >2.5 L/min/m²
- Monitoring
Paralysis Management
- Assumed delayed spinal ischemia:
- Drain CSF to 8-12 mmHg if lumbar drain present.
- Increase MAP by 10 mmHg every 5 minutes until weakness resolves or high MAP reached (Volume and Vasopressors).
- If no improvement in 10 minutes, ready lumbar drain.
- Insert lumbar drain at 20 minutes if not responding, drain to ICP of 10 mmHg.
- If no improvement, perform CT spine to exclude hematoma.
- Wean vasopressors as tolerated following improvement in weakness.
Renal Protection
Background
- Acute Kidney Injury (AKI) is common (10–30%), and dialysis is associated with >50% mortality post-repair.
- Injury from ischemia-reperfusion, embolization, hypoperfusion, and oxidative stress.
Risk Factors
- Suprarenal or supraceliac clamping.
- Prolonged clamp time
- Pre-existing renal impairment.
- Hypotension and low cardiac output post-unclamping.
Key Strategies
- Ischemic Time Minimization
- Reduce cross-clamp duration; prioritize renal arteries for reperfusion.
- Renal Perfusion
- Cold perfusion with 4°C crystalloid into renal arteries.
- Selective distal perfusion via left heart bypass or perfusion shunt.
- Fluid Management
- Preload with balanced fluids before unclamping.
- Avoid hypovolemia and over-diuresis.
- Target urine output >0.5 mL/kg/h.
- Hemodynamic Stability
- MAP ≥80 mmHg during and after surgery.
- Use vasopressors to avoid renal hypoperfusion.
- Avoid hypotension post-unclamp.
- Pharmacologic Adjuncts
- Mannitol 0.5 g/kg before cross-clamp: osmotic diuresis, free radical scavenger.
- Avoid nephrotoxins (e.g., NSAIDs, aminoglycosides).
- No role for low-dose dopamine in AKI prevention.
- Monitoring
- Hourly U/O.
- Serial creatinine and electrolytes.
- Consider early nephrology input for RRT planning.
- Avoiding Secondary Insults
- Minimize hemolysis (cell salvage filter).
- Ensure normothermia.
- Monitor ABG for acidosis and correct promptly.
Heparin Management
- Administer 3000–5000U just before cross-clamp.
- Reverse with protamine 0.5–1 mg per 100U of heparin IV slowly to avoid hypotension.
Aortic Cross-Clamp (AXC)
- Proximal hypertension due to increased SVR, SVC flow, and sympatho-adrenal response.
- Manage with deepened anesthesia, β-blockers (labetalol 5–10 mg), GTN infusion, or epidural LA.
- Metabolic acidosis develops due to ischemic lower limbs.
- Maintain minute ventilation to develop respiratory alkalosis, minimizing metabolic acidosis upon unclamping.
- Check ABGs for Hct, metabolic acidosis, respiratory compensation, and ionized Ca2+.
- Start fluid administration, aiming for moderately increased CVP (5 cmH2O above baseline) by unclamping.
Unclamping
- Gradual release helps CVS stability, reduces sudden hypotension, preserves renal function.
- Hypotension post-unclamping due to decreased SVR, relative hypovolemia, myocardial ‘stunning.’
- Treat with IV fluids, lighten anesthesia, small doses of inotropes (adrenaline 10 µg aliquots), calcium gluconate (up to 10 mL of 10%).
- Use isotonic crystalloid or colloid for fluid replacement.
- Transfuse blood products as needed (Hct < 25%, platelets < 100 × 109/L).
- Check ACT if coagulopathy is suspected; TEG for comprehensive coagulation status.
- TOE for additional information during the cross-clamp period.
Special Considerations
- Epidural Management:
- Bolus epidural morphine 2–3 mg at induction for 12–24 hr analgesia.
- Use epidural LA sparingly until aorta is closed to facilitate treatment of aortic unclamping hypotension.
Distal Aortic Perfusion Options
- Bypass pump commonly used except in type IV aneurysms.
- Techniques:
- Left Heart Bypass: Left atria to left femoral artery, no oxygenator required, heat exchanger for cooling.
- Femoral-Femoral Bypass: Femoral vein cannula to IVC, blood returned to left femoral artery, oxygenator and heat exchanger in circuit.
- Heparinization required (ACT 200-250), less than full CPB due to antithrombotic coating and absence of reservoirs.
- Passive shunt from proximal to distal aorta.
Postoperative Management
- Sedation and ventilation in ICU.
- Ensure adequate cardiac output, perfusion pressures, and O2 delivery.
- Normothermia with active and passive rewarming.
- Continual assessment of volume status and ensuring normovolemia.
- Monitoring for AKI, MI, arrhythmias, and neurological injury.
- PACU/ICU, usually intubated post-op, maintain MAP > 80 mmHg, ongoing CSF drainage for 48 hours.
Complications
- Abdominal Compartment Syndrome:
- Risks: anemia, prolonged hypotension, CPR, hypothermia, severe acidosis (base deficit > 14 mEq), aggressive fluid resuscitation.
- Renal Failure
- Spinal Cord Injury with Paralysis
- The reported incidence of an adverse outcome after TAAA surgery, including renal failure requiring dialysis at hospital discharge, stroke, permanent paraplegia, or paraparesis, is 16%
- Overall operative mortality of 8 to 10%
Management Summaries
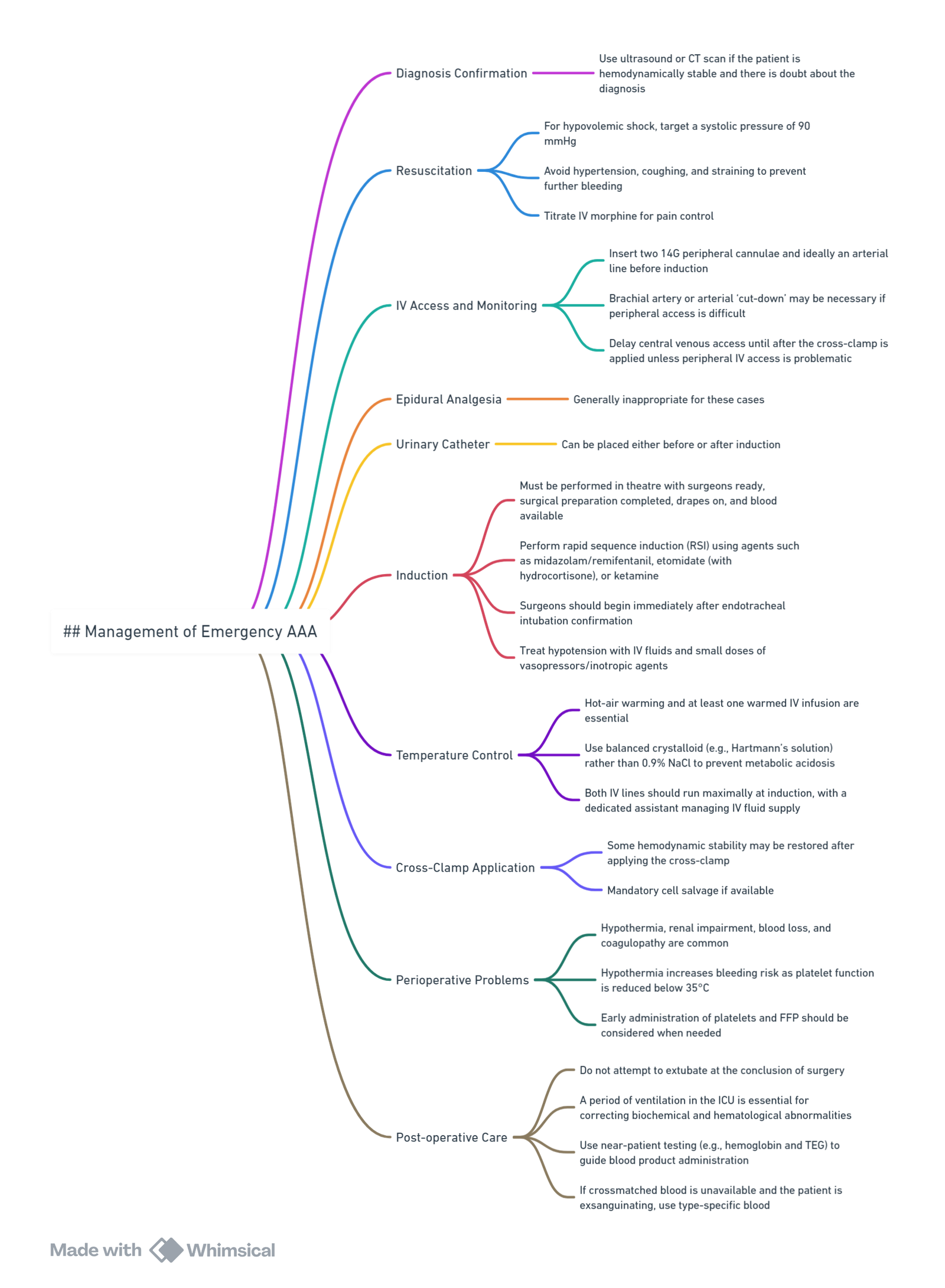
Management follows the protocol for elective AAA repair with specific additional considerations:
Thoraco-abdominal Aortic Aneurysm Repair
Links
Past Exam Questions
Renal Protection in Open Surgical Repair of Abdominal Aortic Aneurysm
With open surgical repair of an abdominal aortic aneurysm, injury to the kidneys remains a major concern.
a) List different methods of preconditioning. Define the time-window in preconditioning. (3)
b) Explain the term post-conditioning. (2)
c) Explain cold renal perfusion. (2)
d) What are the 3 most valuable steps to improve renal outcome? (3)
References:
- Berry, K., Gudgeon, J., & Taylor, J. F. (2022). Anaesthesia for endovascular repair of ruptured abdominal aortic aneurysms. BJA Education, 22(6), 208-215. https://doi.org/10.1016/j.bjae.2022.02.001
- Kothandan H, Haw Chieh GL, Khan SA, Karthekeyan RB, Sharad SS. Anesthetic considerations for endovascular abdominal aortic aneurysm repair. Ann Card Anaesth. 2016 Jan-Mar;19(1):132-41. doi: 10.4103/0971-9784.173029. PMID: 26750684; PMCID: PMC4900395.
- Agarwal S, Kendall J, Quarterman C. Perioperative management of thoracic and thoracoabdominal aneurysms. BJA Education. 2019;19(4):119-125. doi:10.1016/j.bjae.2019.01.004
- Leonard, A. and Thompson, J. P. (2008). Anaesthesia for ruptured abdominal aortic aneurysm. Continuing Education in Anaesthesia Critical Care &Amp; Pain, 8(1), 11-15. https://doi.org/10.1093/bjaceaccp/mkm050
- The Calgary Guide to Understanding Disease. (2024). Retrieved June 5, 2024, from https://calgaryguide.ucalgary.ca/
- FRCA Mind Maps. (2024). Retrieved June 5, 2024, from https://www.frcamindmaps.org/
- Anesthesia Considerations. (2024). Retrieved June 5, 2024, from https://www.anesthesiaconsiderations.com/
Summaries:
Aortic dissection
Aortic dissection-Calgary
AAA complications- Calgary
Aortic dissection-video
Spinal protection-video
Anaesthesia for aortic arch surgery-video
Copyright
© 2025 Francois Uys. All Rights Reserved.
id: “477981c1-8693-43c5-a9fe-9479d2a01e1f”



